Glucose-derived receptors for photo-controlled binding of amino acid esters in water
- PMID: 39972110
- PMCID: PMC11840139
- DOI: 10.1038/s42004-025-01445-x
Glucose-derived receptors for photo-controlled binding of amino acid esters in water
Abstract
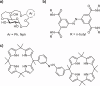
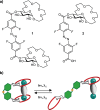
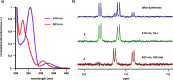
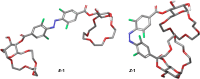
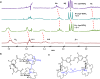
Selective receptors of amino acids in aqueous media are highly sought after as they may enable the creation of novel diagnostic and sensing tools. Photoswitchable receptors are particularly attractive for such purposes as their response and selectivity towards bioanalytes can be modulated using light. Herein we report glucose-based photoswitchable receptors of amino-acid methyl esters and biogenic amines in water. The tetra-ortho-fluoroazobenzene unit in the receptors structure allows to control the distance between their binding sites using light. The Z-isomers of both receptors, having these sites in closer proximity, bind lysine, ornithine and arginine esters significantly stronger compared to E-isomers, where the binding sites are further apart.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Amino Acids: Biochemistry and Nutrition (Ed. Wu, G.), (CRC Press, Boca Raton, 2010).
-
- Cordes, M. et al. Influence of amino acid side chains on long-distance electron transfer in peptides: Electron hopping via “Stepping Stones”. Angew. Chem. Int. Ed.47, 3461–3463 (2008). - PubMed
-
- D’Aniello, S., Somorjai, I., Garcia-Fernàndez, J., Topo, E. & D’Aniello A. D-Aspartic acid is a novel endogenous neurotransmitter. FASEB J25, 1014–1027 (2011). - PubMed
-
- Martin, C. & Zhang, Y. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol.6, 838–849 (2005). - PubMed
-
- Amino-acids, Peptides and Proteins in Organic Chemistry (Ed. Hughes, A. B.), I-V (Wiley-VCH, Weinheim, 2010).
LinkOut - more resources
Full Text Sources

