Targeting fatty acid synthase reduces aortic atherosclerosis and inflammation
- PMID: 39972116
- PMCID: PMC11840040
- DOI: 10.1038/s42003-025-07656-1
Targeting fatty acid synthase reduces aortic atherosclerosis and inflammation
Abstract
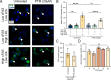
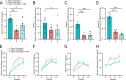
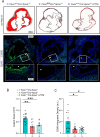
Fatty acid synthase (FAS) is predominantly expressed in the liver and adipose tissue. It plays vital roles in de novo synthesis of saturated fatty acids and regulates insulin sensitivity. We previously demonstrated that serum circulating FAS (cFAS) is a clinical biomarker for advanced atherosclerosis, and that it is conjugated to low-density lipoproteins (LDL). However, it remains unknown whether cFAS can directly impact atheroprogression. To investigate this, we evaluate whether cFAS impacts macrophage foam cell formation - an important cellular process leading to atheroprogression. Macrophages exposed to human serum containing high levels of cFAS show increased foam cell formation as compared to cells exposed to serum containing low levels of cFAS. This difference is not observed using serum containing either high or low LDL. Pharmacological inhibition of cFAS using Platensimycin (PTM) decreases foam cell formation in vitro. In Apoe-/- mice with normal FAS expression, administration of PTM over 16 weeks along with a high fat diet decreases cFAS activity and aortic atherosclerosis without affecting circulating total cholesterol. This effect is also observed in Apoe-/- mice with liver-specific knockout of hepatic Fasn. Reductions in aortic root plaque are associated with decreased macrophage infiltration. These findings demonstrate that cFAS plays an important role in arterial atheroprogression.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: M.Z. is co-founder of AirSeal CardioVascular, Inc., a biomedical startup company that aims to clinically translate diagnostic approaches for individuals suffering from complications related to atherosclerotic cardiovascular disease. All other authors declare no competing interests.
Figures




References
-
- Libby, P. et al. Atherosclerosis. Nat. Rev. Dis. Prim.5, 56 (2019). - PubMed
-
- Song, P. et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob. Health8, e721–e729 (2020). - PubMed
-
- Centers for Disease C, Prevention. Vital signs: prevalence, treatment, and control of high levels of low-density lipoprotein cholesterol--United States, 1999–2002 and 2005-200. MMWR Morb. Mortal. Wkly Rep.60, 109–114 (2011). - PubMed
-
- Baigent, C. et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet366, 1267–1278 (2005). - PubMed
MeSH terms
Substances
Grants and funding
- R01HL150891/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- P41 GM103422/GM/NIGMS NIH HHS/United States
- R01 DK101392/DK/NIDDK NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- R01HL153262/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

