A dual-pathway architecture for stress to disrupt agency and promote habit
- PMID: 39972126
- PMCID: PMC12011321
- DOI: 10.1038/s41586-024-08580-w
A dual-pathway architecture for stress to disrupt agency and promote habit
Abstract
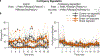
Chronic stress can change how we learn and, thus, how we make decisions1-5. Here we investigated the neuronal circuit mechanisms that enable this. Using a multifaceted systems neuroscience approach in male and female mice, we reveal a dual-pathway, amygdala-striatal neuronal circuit architecture by which a recent history of chronic stress disrupts the action-outcome learning underlying adaptive agency and promotes the formation of inflexible habits. We found that the projection from the basolateral amygdala to the dorsomedial striatum is activated by rewarding events to support the action-outcome learning needed for flexible, goal-directed decision-making. Chronic stress attenuates this to disrupt action-outcome learning and, therefore, agency. Conversely, the projection from the central amygdala to the dorsomedial striatum mediates habit formation. Following stress, this pathway is progressively recruited to learning to promote the premature formation of inflexible habits. Thus, stress exerts opposing effects on two amygdala-striatal pathways to disrupt agency and promote habit. These data provide neuronal circuit insights into how chronic stress shapes learning and decision-making, and help understanding of how stress can lead to the disrupted decision-making and pathological habits that characterize substance use disorders and mental health conditions.
© 2025. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
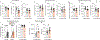



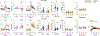

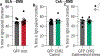




Update of
-
A dual-pathway architecture enables chronic stress to disrupt agency and promote habit formation.bioRxiv [Preprint]. 2024 Sep 24:2023.10.03.560731. doi: 10.1101/2023.10.03.560731. bioRxiv. 2024. Update in: Nature. 2025 Apr;640(8059):722-731. doi: 10.1038/s41586-024-08580-w. PMID: 37873076 Free PMC article. Updated. Preprint.

