SPO11 dimers are sufficient to catalyse DNA double-strand breaks in vitro
- PMID: 39972130
- PMCID: PMC11922746
- DOI: 10.1038/s41586-024-08574-8
SPO11 dimers are sufficient to catalyse DNA double-strand breaks in vitro
Abstract
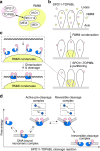
SPO11 initiates meiotic recombination through the induction of programmed DNA double-strand breaks (DSBs)1,2, but this catalytic activity has never been reconstituted in vitro3,4. Here, using Mus musculus SPO11, we report a biochemical system that recapitulates all the hallmarks of meiotic DSB formation. We show that SPO11 catalyses break formation in the absence of any partners and remains covalently attached to the 5' broken strands. We find that target site selection by SPO11 is influenced by the sequence, bendability and topology of the DNA substrate, and provide evidence that SPO11 can reseal single-strand DNA breaks. In addition, we show that SPO11 is monomeric in solution and that cleavage requires dimerization for the reconstitution of two hybrid active sites. SPO11 and its partner TOP6BL form a 1:1 complex that catalyses DNA cleavage with an activity similar to that of SPO11 alone. However, this complex binds DNA ends with higher affinity, suggesting a potential role after cleavage. We propose a model in which additional partners of SPO11 required for DSB formation in vivo assemble biomolecular condensates that recruit SPO11-TOP6BL, enabling dimerization and cleavage. Our work establishes SPO11 dimerization as the fundamental mechanism that controls the induction of meiotic DSBs.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures












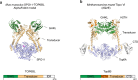

References
-
- Keeney, S., Giroux, C. N. & Kleckner, N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell88, 375–384 (1997). - PubMed
-
- Bergerat, A. et al. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature386, 414–417 (1997). - PubMed
-
- de Massy, B. Initiation of meiotic recombination: how and where? Conservation and specificities among eukaryotes. Annu. Rev. Genet.47, 563–599 (2013). - PubMed
-
- Robert, T., Vrielynck, N., Mezard, C., de Massy, B. & Grelon, M. A new light on the meiotic DSB catalytic complex. Semin. Cell Dev. Biol.54, 165–176 (2016). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

