Paracrine FGF21 dynamically modulates mTOR signaling to regulate thymus function across the lifespan
- PMID: 39972173
- PMCID: PMC12003089
- DOI: 10.1038/s43587-024-00801-1
Paracrine FGF21 dynamically modulates mTOR signaling to regulate thymus function across the lifespan
Abstract
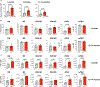
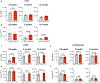
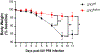
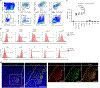
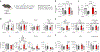
Consequences of age-associated thymic atrophy include declining T-cell responsiveness to pathogens and vaccines and diminished T-cell self-tolerance. Cortical thymic epithelial cells (cTECs) are primary targets of thymic aging, and recent studies suggested that their maintenance requires mTOR signaling downstream of medullary TEC (mTEC)-derived growth factors. Here, to test this hypothesis, we generated a knock-in mouse model in which FGF21 and mCherry are expressed by most mTECs. We find that mTEC-derived FGF21 promotes temporally distinct patterns of mTORC1 and mTORC2 signaling in cTECs, promotes thymus and individual cTEC growth and maintenance, increases T-cell responsiveness to viral infection, and diminishes indicators of peripheral autoimmunity in older mice. The effects of FGF21 overexpression on thymus size and mTOR signaling were abrogated by treatment with the mTOR inhibitor rapamycin. These results reveal a mechanism by which paracrine FGF21 signaling regulates thymus size and function throughout the lifespan, as well as potential therapeutic targets for improving T-cell function and tolerance in aging.
© 2025. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

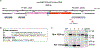
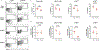










References
-
- Petrie HT & Zuniga-Pflucker JC Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu. Rev. Immunol 25, 649–679 (2007). - PubMed
MeSH terms
Substances
Grants and funding
- R01AG086271/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 AG086271/AG/NIA NIH HHS/United States
- P30 AG013319/AG/NIA NIH HHS/United States
- T32 AI138944/AI/NIAID NIH HHS/United States
- T32AI138944/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R21 AI154109/AI/NIAID NIH HHS/United States
- S10 OD030432/OD/NIH HHS/United States
- R21 AG081709/AG/NIA NIH HHS/United States
- R21AI154109R21/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- P30 CA054174/CA/NCI NIH HHS/United States
- R21AG081709/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

