Patient preferences for long-acting HIV treatment: a preference heterogeneity assessment
- PMID: 39972307
- PMCID: PMC11841254
- DOI: 10.1186/s12879-025-10546-w
Patient preferences for long-acting HIV treatment: a preference heterogeneity assessment
Abstract
Background: Long-acting antiretroviral therapy (LA-ART) is an emerging alternative to daily oral ART pills that may improve HIV treatment adherence. We studied preference heterogeneity for LA-ART among people with HIV (PWH) in western Washington State and Atlanta, Georgia to determine how preference heterogeneity was related to individual characteristics.
Methods: We recruited 699 PWH to complete a survey including 17 choice-tasks, each of which included two hypothetical LA-ART alternatives and current daily oral therapy. Each hypothetical alternative was defined by mode (long-acting [LA] oral pills, subcutaneous injections, intramuscular injections, and implants), frequency, treatment location (home, clinic, or pharmacy), injection pain, pre-treatment time undetectable, pre-treatment reaction testing, and late-dose leeway. We fitted a latent class model to the data and investigated associations between class membership and individual characteristics.
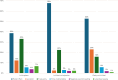
Results: Our sample had three classes which were defined by their treatment preferences. Two classes preferred LA-ART over current treatment: the LA-Implant class (29%) and the LA-Oral-or-Injection class (35%). In contrast, the Daily-or-LA-Oral class (36%) preferred current treatment or LA oral pills taken at home. Compared to the third class, participants from the other two were younger, more educated, less adherent to current ART, and less averse to injections. Further, LA-Implant participants were less likely to be virally suppressed and had easier clinic access. LA-Oral-or-Injection participants had a higher prevalence of psychotic disorders.
Conclusion: These results provide a deeper understanding of the preference landscape for LA-ART and can aid in the development of interventions better aligned with individual preferences.
Keywords: Discrete choice experiment; Long-acting ART; Preference heterogeneity; Preferences elicitation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The UW and Emory University both approved the study protocols and informed consent documents, and the UW served as the institutional review board (IRB) of record (STUDY00007390). All participants provided electronic informed consent. Consent for publication: All participants provided electronic consent. Competing interests: BH is an employee of Pfizer, but Pfizer had no input or influence on the study. SMG has received support from Gilead and Cepheid for her research. VCM has received investigator-initiated research grants (to the institution) and consultation fees (both unrelated to the current work) from Eli Lilly, Bayer, Merck, Gilead Sciences, and ViiV. The other authors declare that they have no conflicts of interest directly relevant to the content of this article.
Figures

References
-
- Centers for Disease Control and Prevention (CDC). HV surveillance supplemental report: estimated HIV incidence and prevalence in the United States, 2018–2022. 2024.
-
- Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

