Interaction between APOE Ɛ4 status, chemotherapy and endocrine therapy on cognitive functioning among breast cancer survivors: the CANTO-Cog longitudinal study
- PMID: 39972319
- PMCID: PMC11841345
- DOI: 10.1186/s13058-025-01974-2
Interaction between APOE Ɛ4 status, chemotherapy and endocrine therapy on cognitive functioning among breast cancer survivors: the CANTO-Cog longitudinal study
Abstract
Background: Apolipoprotein Ɛ4 genotype (APOE4) has been associated with cancer-related cognitive impairment, but its interaction with treatments remains unclear. This longitudinal study aims to evaluate the association between APOE4 and cognitive impairment in women with breast cancer (BC) undergoing chemotherapy (CT) or endocrine therapy (ET).
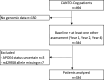
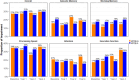
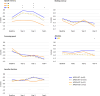
Findings: Patients with stage I-III breast cancer completed cognitive tests at diagnosis (before surgery), then at year-1, year-2, and year-4 post-diagnosis. APOE4 status (APOE4+ [carriers] vs. APOE4- [non-carriers]) was genotyped from blood sample. Cognitive outcomes included episodic memory, working memory, attention, processing speed, and executive functions. Patients were defined as having overall cognitive impairment if ≥ 2 domains were impaired. We fitted logistic and linear mixed models to assess associations of APOE4 status with cognitive impairment over time and interactions of APOE4 with CT and ET. Among 334 patients, 64 (19%) were APOE4+, 117 (35%) patients were treated with CT, 41 (12%) with ET, and 162 (49%) with CT+ET. There were no significant association between overall cognitive impairment and APOE4, nor interactions with CT or ET. At year-4, APOE4+ patients treated with ET had lower attention performance than APOE4- patients not treated with ET, and APOE4+ patients not treated with ET had lower episodic memory performance than APOE4- patients not treated with ET.
Conclusions: This study suggests APOE4 genotyping is ineffective for detecting cognitive impairment in BC. New genotypes should be identified to predict cognitive decline in BC.
Keywords: APOE4; Breast cancer; Cancer-related cognitive impairment; Chemotherapy; Endocrine therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All participants provided written informed consent and the study was approved by the ethics committee (ID-RCB:2011-A01095-36,11-039). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Lange M, Hardy-Léger I, Licaj I, Pistilli B, Rigal O, Le Fel J, et al. Cognitive impairment in patients with breast cancer before surgery: results from a CANTO cohort subgroup. Cancer Epidemiol Biomark Prev. 2020;29:1759–66. - PubMed
-
- Sleurs C, Amidi A, Wu LM, Kiesl D, Zimmer P, Lange M, et al. Cancer-related cognitive impairment in non-CNS cancer patients: targeted review and future action plans in Europe. Crit Rev Oncol Hematol. 2022;180:103859. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

