Alanine and glutathione targeting of dopamine- or ibuprofen-coupled polypeptide nanocarriers increases both crossing and protective effects on a blood-brain barrier model
- PMID: 39972353
- PMCID: PMC11837687
- DOI: 10.1186/s12987-025-00623-2
Alanine and glutathione targeting of dopamine- or ibuprofen-coupled polypeptide nanocarriers increases both crossing and protective effects on a blood-brain barrier model
Abstract
Background: Targeting the blood-brain barrier (BBB) is a key step for effective brain delivery of nanocarriers. We have previously discovered that combinations of BBB nutrient transporter ligands alanine and glutathione (A-GSH), increase the permeability of vesicular and polypeptide nanocarriers containing model cargo across the BBB. Our aim was to investigate dopamine- and ibuprofen-coupled 3-armed poly(L-glutamic acid) nanocarriers targeted by A-GSH for transfer across a novel human co-culture model with induced BBB properties. In addition, the protective effect of ibuprofen containing nanoparticles on cytokine-induced barrier damage was also measured.
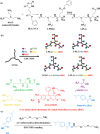
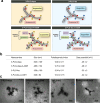
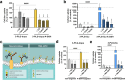
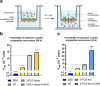
Method: Drug-coupled nanocarriers were synthetized and characterized by dynamic light scattering and transmission electron microscopy. Cellular effects, uptake, and permeability of the nanoparticles were investigated on a human stem cell-based co-culture BBB model with improved barrier properties induced by a small molecular cocktail. The model was characterized by immunocytochemistry and permeability for marker molecules. Nanocarrier uptake in human brain endothelial cells and midbrain organoids was quantified by spectrofluorometry and visualized by confocal microscopy. The mechanisms of cellular uptake were explored by addition of free targeting ligands, endocytic and metabolic inhibitors, co-localization of nanocarriers with intracellular organs, and surface charge modification of cells. The protective effect of ibuprofen-coupled nanocarriers was investigated against cytokine-induced barrier damage by impedance and permeability measurements.
Results: Targeted nanoformulations of both drugs showed elevated cellular uptake in a time-dependent, active manner via endocytic mechanisms. Addition of free ligands inhibited the cellular internalization of targeted nanocarriers suggesting the crucial role of ligands in the uptake process. A higher permeability across the BBB model was measured for targeted nanocarriers. After crossing the BBB, targeted dopamine nanocarriers subsequently entered midbrain-like organoids derived from healthy and Parkinson's disease patient-specific stem cells. The ibuprofen-coupled targeted nanocarriers showed protective effects against cytokine-induced barrier damage.
Conclusion: BBB-targeted polypeptide nanoparticles coupled to therapeutic molecules were effectively taken up by brain organoids or showing a BBB protective effect indicating potential applications in nervous system pathologies.
Keywords: Alanine; Blood–brain barrier; Dopamine; Dual-targeted nanocarriers; Glutathione; Human stem cell derived endothelial cell; Ibuprofen; In vitro model; Poly(l-glutamic acid).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experiments with brain organoids were performed in accordance with the national and international ethics guidelines, and were approved by the ethics committee at the University of Luxembourg, Comité National d’Ethique de Recherche (CNER; approval code 201901/01, date of approval: 2 May 2019). Consent for publication: All authors have read and consented to the publication of the manuscript. Competing interests: The authors declare no competing interests.
Figures






References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37(1):13–25. 10.1016/j.nbd.2009.07.030. - PubMed
-
- Abbott NJ. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36(3):437–49. 10.1007/s10545-013-9608-0. - PubMed
-
- Bartels AL, Leenders KL. Parkinson’s disease: the syndrome, the pathogenesis and pathophysiology. Cortex. 2009;45(8):915–21. 10.1016/j.cortex.2008.11.010. - PubMed
-
- Deli MA. Drug transport and the blood-brain barrier. In: Tihanyi K, Vastag M, editors. Solubility, delivery, and ADME problems of drugs and drug-candidates. Bentham Science Publishers Ltd., Washington; 2011. p. 144–165.
MeSH terms
Substances
Grants and funding
- PD 138930/National Research, Development and Innovation Office, Budapest, Hungary
- ÚNKP-23-3-SZTE-535/New National Excellence Program of the Ministry for Innovation and Technology
- ÚNKP-23-3-SZTE-315/New National Excellence Program
- EKÖP-393/Egyetemi Kutatói Ösztöndíj Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund
- SA-111/2021/Hungarian Research Network
- NSTC107-2923-M-006-002-MY3 (M-ERA.NET2 nanoPD)/National Science Technology Council, Taiwan
- 143233/Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the FK_22 funding scheme
- NNE-29617 (M-ERA.NET2 nanoPD)/National Research, Development and Innovation Office of Hungary
LinkOut - more resources
Full Text Sources

