An index of the initial blood pressure response to angiotensin II treatment and its association with clinical outcomes in vasodilatory shock
- PMID: 39972379
- PMCID: PMC11837372
- DOI: 10.1186/s13054-025-05311-z
An index of the initial blood pressure response to angiotensin II treatment and its association with clinical outcomes in vasodilatory shock
Abstract
Background: No standardized index exists to assess cardiovascular responsiveness to angiotensin-II. We hypothesized that a standardized index of initial blood pressure response to angiotensin-II treatment would be associated with clinical outcomes.
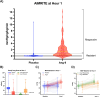
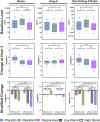
Methods: Using data from the Angiotensin Therapy for High Output Shock (ATHOS-3) trial, we developed an Angiotensin-II Initial MAP Response Index of Treatment Effect (AIMRITE) defined as (MAP at hr1 - MAP at baseline)/study drug dose. We assessed AIMRITE continuously and, based on observed distributions, we additionally categorized patients as "responsive" or "resistant", with responsiveness defined by an AIMRITE ≥ 0.90 mmHg/ng/kg/min. The primary clinical outcome was 28-day mortality. Secondary outcomes included days alive and vasopressor- or ventilator- or renal replacement therapy-free at day-7. Biological outcomes included baseline renin, angiotensin-II, and renin/angiotensin-II ratio, and their change at hr3.
Results: Of 158 placebo patients, as expected, 157 (99%) had AIMRITE < 0.90 mmHg/ng/kg/min (median AIMRITE 0.02; IQR - 0.03-0.10). In contrast, 163 patients assigned to angiotensin-II had a median AIMRITE of 1.43 mmHg/ng/kg/min (IQR 0.35-2.83). Of these, 97 (60%) were responsive (median AIMRITE 2.55; IQR 1.66-4.12) and 66 (40%) were resistant (median AIMRITE 0.24; IQR 0.10-0.52). Each 1.0-unit increase in AIMRITE was associated with a 16% lower hazard of death (HR: 0.84 per-mmHg/ng/kg/min [95% CI 0.74-0.95], p = 0.0062). Responsive patients had half the mortality hazard than resistant patients (HR: 0.50 [95% CI 0.32-0.78], p = 0.0026) and placebo patients (HR 0.58 [95% CI 0.40-0.86], p = 0.0064). Resistant patients had a similar mortality hazard to placebo (HR 1.17 [95% CI 0.80-1.72], p = 0.41). Compared to resistant patients, responsive patients had lower baseline renin and renin/angiotensin-II ratio, but a greater decrease in both at hr3. When stratified by baseline renin level, mortality was highest in placebo patients with high renin (69%) and angiotensin-II resistant patients with low renin (61%).
Conclusions: Among patients with catecholamine-refractory vasodilatory shock treated with angiotensin-II, the AIMRITE was associated with mortality at day-28. Responsive angiotensin-II patients had higher survival versus both angiotensin-II resistant patients and those treated with placebo plus standard vasopressors. This index may serve as a prognostic indicator and early identifier of patients most likely to benefit from angiotensin-II.
Trial registration: ClinicalTrials.gov NCT02338843.
Keywords: Angiotensin II; Norepinephrine; Renin-angiotensin system; Septic; Shock.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The trial was conducted in accordance with Good Clinical Practice guidelines, applicable local regulations, and Declaration of Helsinki principles. The respective independent institutional review boards reviewed the protocol, informed-consent form, and all other documents before study initiation. Competing interests: DEL, LWB, KAH, AKK, MO, and MTC declare no competing interests. RB has received consulting fees from both Innoviva and Viatris as well as research grants from Innoviva. DRH, CDA, and TNH are employees of Innoviva Specialty Therapeutics, Inc., of which La Jolla Pharmaceutical Company is an affiliate. LSC declares that he was formerly an employee of LJPC. PMW is a consultant for Wolters Kluwer/UpToDate and previously received consulting fees from Viatris.
Figures



References
-
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–143. 10.1097/CCM.0000000000005337. - PubMed
-
- De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779–89. 10.1056/NEJMoa0907118. - PubMed
-
- Stolk RF, van der Poll T, Angus DC, van der Hoeven JG, Pickkers P, Kox M. Potentially inadvertent immunomodulation: norepinephrine use in sepsis. Am J Respir Crit Care Med. 2016;194(5):550–8. 10.1164/rccm.201604-0862CP. - PubMed
-
- Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877–87. 10.1056/NEJMoa067373. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

