Cornuside alleviates cognitive impairments induced by Aβ1-42 through attenuating NLRP3-mediated neurotoxicity by promoting mitophagy
- PMID: 39972387
- PMCID: PMC11837312
- DOI: 10.1186/s13195-025-01695-w
Cornuside alleviates cognitive impairments induced by Aβ1-42 through attenuating NLRP3-mediated neurotoxicity by promoting mitophagy
Erratum in
-
Correction: Cornuside alleviates cognitive impairments induced by Aβ1-42 through attenuating NLRP3-mediated neurotoxicity by promoting mitophagy.Alzheimers Res Ther. 2025 Mar 19;17(1):63. doi: 10.1186/s13195-025-01715-9. Alzheimers Res Ther. 2025. PMID: 40108656 Free PMC article. No abstract available.
Abstract
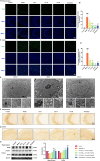
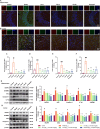
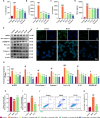
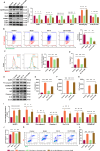
Alzheimer's disease (AD) is a progressive neurodegenerative disorder in which mitochondrial dysfunction and neuroinflammation play crucial roles in its progression. Our previous studies found that cornuside from Cornus officinalis Sieb.Et Zucc is an anti-AD candidate, however, its underlying mechanism remains unknown. In the present study, AD mice were established by intracerebroventricular injection of Aβ1-42 and treated with cornuside (3, 10, 30 mg/kg) for 2 weeks. Cornuside significantly ameliorated behavioral deficits, protected synaptic plasticity and relieved neuronal damage in Aβ1-42 induced mice. Importantly, cornuside decreased NLRP3 inflammasome activation, characterized by decreased levels of NLRP3, ASC, Caspase-1, GSDMD, and IL-1β. Furthermore, cornuside promoted mitophagy accompanied by decreasing SQSTM1/p62 and promoting LC3B-I transforming into LC3B-II, via Pink1/Parkin signaling instead of FUNDC1 or BNIP3 pathways. In order to investigate the relationship between NLRP3 inflammasome and mitophagy in the neuroprotective mechanism of cornuside, we established an in-vitro model in BV2 cells exposed to LPS and Aβ1-42. And cornuside inhibited NLRP3 inflammasome activation and subsequent cytokine release, also protected neurons from damaging factors in microenvironment of conditional culture. Cornuside improved mitochondrial function by promoting oxidative phosphorylation and glycolysis, decreasing the production of ROS and mitochondrial membrane potential depolarization. Besides, mitophagy was also facilitated with increased colocalization of MitoTracker with LC3B and Parkin, and Pink1/Parkin, FUNDC1 and BNIP3 pathways were all involved in the mechanism of cornuside. By blocking the formation of autophagosomes by 3-MA, the protective effects on mitochondria, the inhibition on NLRP3 inflammasome as well as neuronal protection in conditional culture were eliminated. There is reason to believe that the promotion of mitophagy plays a key role in the NLRP3 inhibition of cornuside. In conclusion, cornuside re-establishes the mitophagy flux which eliminates damaged mitochondria and recovers mitochondrial function, both of them are in favor of inhibiting NLRP3 inflammasome activation, then alleviating neuronal and synaptic damage, and finally improving cognitive function.
Keywords: Alzheimer’s disease; Cornuside; Mitophagy; NLRP3; Neuroprotection.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal procedures were approved by the Animal Care and Use Committee of China-Japan Friendship Hospital. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures






References
-
- Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25:59–70. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. - PubMed
-
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular abeta and synaptic dysfunction. Neuron. 2003;39:409–21. - PubMed
MeSH terms
Substances
Grants and funding
- 82404883/National Natural Science Foundation of China
- 82273815/National Natural Science Foundation of China
- 82273809/National Natural Science Foundation of China
- ZRJY2023-QM10/National High Level Hospital Clinical Research Funding & Elite Medical Professionals Project of China-Japan Friendship Hospital
- ZRJY2023-QM28/National High Level Hospital Clinical Research Funding & Elite Medical Professionals Project of China-Japan Friendship Hospital
- ZRJY2024-BJ01/National High Level Hospital Clinical Research Funding & Elite Medical Professionals Project of China-Japan Friendship Hospital
- 2024-NHLHCRF-JBGS-WZ-07/National High Level Hospital Clinical Research Funding & Elite Medical Professionals Project of China-Japan Friendship Hospital
- 3332023096/Central Universities Fundamental for Basic Scientific Research of Peking Union Medical College
LinkOut - more resources
Full Text Sources
Miscellaneous

