A Case Series and Literature Review on Zanubrutinib Therapy for the Treatment of Relapsed/Refractory Immune Thrombocytopenia
- PMID: 39973148
- PMCID: PMC11840241
- DOI: 10.1002/cnr2.70152
A Case Series and Literature Review on Zanubrutinib Therapy for the Treatment of Relapsed/Refractory Immune Thrombocytopenia
Abstract
Background: Immune thrombocytopenia (ITP) is an acquired autoimmune disease characterised by low platelet count. Treatment discontinuation or heterogeneity in the pathogenesis of ITP heightens the occurrence of relapsed or refractory ITP. Bruton's tyrosine kinase (BTK) has emerged as a promising target for autoimmune disorders.
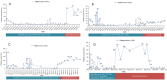
Case: In this case series, we have explored the efficacy and safety of Bruton's tyrosine kinase inhibitors (BTKi) in treating relapsed/refractory ITP, by retrospective analysis of the diagnostic history and efficacy of four patients with relapsed/refractory ITP who attended the Affiliated Cancer Hospital of Zhengzhou University and were treated with BTKi. All four patients received > 4 lines of ITP treatment and did not respond to splenectomy or other interventions before and after treatment with BTK inhibitor. After adjusting to the treatment with BTKi, one patient achieved a complete response, and two patients achieved a partial response. All three patients achieved sustained remission with platelet counts of > 50 × 109/L maintained for 1045, 390 and 334 days, respectively. Another patient died of intracranial haemorrhage due to a decline in the platelet count after discontinuation of the drug, and the duration of sustained remission before discontinuation of the drug was 120 days. Four patients had no significant abnormalities in the functions of the liver and kidney monitored during the treatment period.
Conclusion: For patients with relapsed/refractory ITP, BTK inhibitor therapy can be considered as an option, with promising preliminary efficacy and safety. However, more clinical trials are needed to verify the exact data.
Keywords: BTK inhibitor; immune thrombocytopenia; refractory; relapse.
© 2025 The Author(s). Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Rilzabrutinib for the Treatment of Immune Thrombocytopenia.Eur J Haematol. 2025 Jul;115(1):4-15. doi: 10.1111/ejh.14425. Epub 2025 Apr 13. Eur J Haematol. 2025. PMID: 40222822 Free PMC article. Review.
-
Efficacy and Safety of the Bruton's Tyrosine Kinase Inhibitor Zanubrutinib in Immune Thrombocytopenia.Am J Hematol. 2025 Aug;100(8):1314-1322. doi: 10.1002/ajh.27718. Epub 2025 May 20. Am J Hematol. 2025. PMID: 40391880 Clinical Trial.
-
Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study.J Hematol Oncol. 2020 May 11;13(1):48. doi: 10.1186/s13045-020-00884-4. J Hematol Oncol. 2020. PMID: 32393328 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Syk and BTK Inhibitors in Immune Thrombocytopenia: A Comprehensive Review of Emerging Evidence.Mediators Inflamm. 2025 May 9;2025:5578929. doi: 10.1155/mi/5578929. eCollection 2025. Mediators Inflamm. 2025. PMID: 40385351 Free PMC article. Review.
-
Treatment of Patients with Relapsed or Refractory Mantle-Cell Lymphoma with Zanubrutinib, a Selective Inhibitor of Bruton's Tyrosine Kinase.Clin Cancer Res. 2020 Aug 15;26(16):4216-4224. doi: 10.1158/1078-0432.CCR-19-3703. Epub 2020 May 27. Clin Cancer Res. 2020. PMID: 32461234 Clinical Trial.
Cited by
-
Rilzabrutinib for the Treatment of Immune Thrombocytopenia.Eur J Haematol. 2025 Jul;115(1):4-15. doi: 10.1111/ejh.14425. Epub 2025 Apr 13. Eur J Haematol. 2025. PMID: 40222822 Free PMC article. Review.
References
-
- Thrombosis and Hemostasis Group, Chinese Society of Hematology, Chinese Medical Association , “Chinese Guideline on the Diagnosis and Management of Adult Primary Immune Thrombocytopenia (Version 2020),” Zhonghua Xue Ye Xue Za Zhi Zhonghua Xueyexue Zazhi 41, no. 8 (2020): 617–623, 10.3760/cma.j.issn.0253-2727.2020.08.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources