Epidemiology, resource use, and treatment patterns of locally advanced or metastatic urothelial carcinoma in France
- PMID: 39973175
- PMCID: PMC11881851
- DOI: 10.1080/14796694.2025.2459058
Epidemiology, resource use, and treatment patterns of locally advanced or metastatic urothelial carcinoma in France
Abstract
Aim: Describe real-world epidemiology, treatment patterns, health care resource utilization, and costs of locally advanced or metastatic urothelial carcinoma (la/mUC) in France.
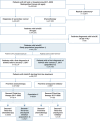
Patients & methods: Retrospective study including all adults with la/mUC diagnosis during January 2017 to December 2020 in the PMSI database.
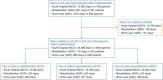
Results: Annual prevalence and incidence ranged from 36.4 to 38.9 and 16.4 to 18.5 cases per 100,000 people, respectively. Of the 25,314 patients with incident la/mUC, 37.6% did not receive first-line systemic treatment. Of the 14,656 patients who started first-line systemic treatment, 66.6%, 22.5%, and 10.9% received 1, 2, and 3 lines of therapy, respectively. Annual per-patient costs in second-/third-line setting ranged from €8803 to €16,012.
Conclusion: The substantial disease burden of la/mUC in France highlights the unmet need for new therapies.
Keywords: France; Incidence; costs; prevalence; treatment; urothelial carcinoma.
Plain language summary
What is this article about?Urothelial carcinoma (UC) is a type of cancer affecting the urinary system. It can spread to other parts of the body, described as locally advanced or metastatic (la/m). We used information from a French database recording hospitalizations in France to find out how many people have la/mUC, how many new cases develop each year, what treatments they receive, how many die in the hospital, and how much their care costs.What were the results?Based on database information, 37 to 39 of every 100,000 people have la/mUC and 17 to 19 of every 100,000 people are identified with a new case yearly. Slightly more than one-third of patients with la/mUC did not receive recommended treatment (chemotherapy) when first diagnosed. Chemotherapy was the most common treatment type for the first, second, or third treatment; checkpoint inhibitors (a unique treatment) became more commonly used as a second treatment over time. Yearly in-hospital death rates were high, ranging from 47.8% of patients who died within 1 year from diagnosis to 62.9% dying within 3 years. Yearly cost of care was high (costing €8803 to €16,012) in patients starting a second or third treatment.What do the results of the study mean?The study shows many patients may not be fit enough or choose not to receive treatment. Even those receiving treatment are at high risk for poor outcomes. The burden of la/mUC in France is high, underscoring the need for more therapies and better supportive care early in disease management.
Conflict of interest statement
Florence Joly reports consulting fees from GSK; payment or honoraria for lectures and presentations (symposium) from 3A, Amgen, Astellas, AstraZeneca, GSK, Ipsen, MSD/ESAI, and Pfizer; support for attending meetings and/or travel from GSK, Ipsen, MSD/ESAI, and Pfizer; participating on an Advisory Board for AstraZeneca, Bayer, GSK, Ipsen, MSD/ESAI, and Pfizer; as well as an unpaid leadership or fiduciary role for GCIG. Stephane Culine reports consulting fees from Bayer; payment or honoraria from AstraZeneca, Ipsen, Janssen, Merck, MSD, and Takeda; support for attending meetings and/or travel from Janssen; and participating on a Data Safety Monitoring Board or Advisory Board for Amgen and BMS. Morgan Roupret reports consulting fees from Astellas, Bayer, BMS, Ipsen, and Janssen. Aurore Tricotel, Emilie Casarotto, and Sandrine Brice are employees of IQVIA, contracted by Astellas Pharma Inc. to conduct the study. Rafael Minacori, Marthe Vuillet, and Marie-Catherine Thomas are employees of Astellas Pharma France. Kirsten Leyland, Anil Upadhyay, Vicki Munro, and Torsten Strunz-McKendry are employees of Astellas Pharma Europe Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support was provided by Catherine Mirvis, BA, and Beth Lesher, PharmD, BCPS, from OPEN Health and was funded by Astellas Pharma Inc. and Pfizer.
Figures



Similar articles
-
Epidemiology and treatment patterns for locally advanced or metastatic urothelial carcinoma: a systematic literature review and gap analysis.J Manag Care Spec Pharm. 2021 Feb;27(2):240-255. doi: 10.18553/jmcp.2020.20285. Epub 2020 Dec 23. J Manag Care Spec Pharm. 2021. PMID: 33355035 Free PMC article.
-
Real-world survival and economic burden among patients with locally advanced or metastatic urothelial carcinoma in the United States.Urol Oncol. 2025 Mar;43(3):189.e9-189.e18. doi: 10.1016/j.urolonc.2024.11.010. Epub 2024 Dec 14. Urol Oncol. 2025. PMID: 39675951
-
Real-world epidemiology and treatment patterns of patients with locally advanced or metastatic urothelial carcinoma: Retrospective analysis of Diagnosis Procedure Combination claims data in Japan.Int J Urol. 2024 Jul;31(7):730-738. doi: 10.1111/iju.15450. Epub 2024 Mar 12. Int J Urol. 2024. PMID: 38468564 Free PMC article.
-
Treatment Patterns, Healthcare Resource Utilization, and Costs Among Patients Diagnosed With Locally Advanced/Metastatic Urothelial Carcinoma Prior to Receiving Enfortumab Vedotin: A Real-World Evidence Study.Int J Urol. 2025 Jun;32(6):650-657. doi: 10.1111/iju.70023. Epub 2025 Mar 5. Int J Urol. 2025. PMID: 40040557 Free PMC article.
-
Epidemiology, treatments, and related biomarkers of locally advanced or metastatic urothelial carcinoma in Chinese population: A scoping review.Cancer Med. 2023 Jul;12(14):15384-15403. doi: 10.1002/cam4.6112. Epub 2023 Jun 30. Cancer Med. 2023. PMID: 37387501 Free PMC article.
Cited by
-
Treatment patterns in patients with locally advanced and metastatic bladder cancer in Denmark 2015-2023 - an updated analysis.Acta Oncol. 2025 May 7;64:630-632. doi: 10.2340/1651-226X.2025.43484. Acta Oncol. 2025. PMID: 40336179 Free PMC article. No abstract available.
References
-
- Berdik C. Unlocking bladder cancer. Nature. 2017;551(7679):S34–S35. - PubMed
-
- Witjes JA, Bruins HM, Carrión A, et al. Muscle-invasive and metastatic bladder cancer [Internet]. Arnhem, The Netherlands: European Association of Urology; [cited 2022 Jul 8]. Available from: https://uroweb.org/guidelines/muscle-invasive-and-metastatic-bladder-cancer
-
• Updated 2022 guidelines for bladder cancer from the European Association of Urology (EAU).
-
- Neuzillet Y, Audenet F, Loriot Y, et al. French AFU cancer committee guidelines – update 2022–2024: muscle-invasive bladder cancer (MIBC). Prog Urol. 2022;32(15):1141–1163. - PubMed
-
• 2022–2024 recommendations for muscle-invasive bladder cancer from the Comité de cancérologie de l’Association Française d’Urologie (CCAFU).
-
- Powles T, Bellmunt J, Comperat E, et al. Bladder cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(3):244–258. - PubMed
-
• Updated 2022 guidelines for bladder cancer from the European Society for Medical Oncology (ESMO).
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
