Merging Photoexcited Nitroarenes with Lewis Acid Catalysis for the Anti-Markovnikov Oxidation of Alkenes
- PMID: 39973366
- PMCID: PMC11877499
- DOI: 10.1021/acs.orglett.5c00389
Merging Photoexcited Nitroarenes with Lewis Acid Catalysis for the Anti-Markovnikov Oxidation of Alkenes
Abstract
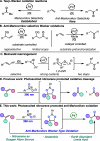
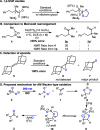
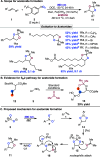
Herein we describe the oxidation of alkenes to carbonyls and acetonides via the interplay of photoexcited nitroarenes and Lewis acid catalysis. A wide range of alkenes were oxidized to aldehyde and ketone products with anti-Markovnikov selectivity and to acetonides when acetone was employed as a co-solvent. Mechanistic studies support that Lewis acid coordination to the 1,3,2-dioxazolidine intermediate results in a 1,2-shift to generate carbonyl derivatives and a nucleophilic substitution pathway for the formation of acetonides.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Rajagopalan A.; Lara M.; Kroutil W. Oxidative Alkene Cleavage by Chemical and Enzymatic Methods. Adv. Syn. Catal. 2013, 355, 3321–3335. 10.1002/adsc.201300882. - DOI
- Campbell A. N.; Stahl S. S. Overcoming the “Oxidant Problem”: Strategies to Use O2 as the Oxidant in Organometallic C–H Oxidation Reactions Catalyzed by Pd (and Cu). Acc. Chem. Res. 2012, 45, 851–863. 10.1021/ar2002045. - DOI - PMC - PubMed
- Shi Z.; Zhang C.; Tang C.; Jiao N. Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant. Chem. Soc. Rev. 2012, 41, 3381–3430. 10.1039/c2cs15224j. - DOI - PubMed
-
- Smidt J.; Hafner W.; Jira R.; Sieber R.; Sedlmeier J.; Sabel A. The Oxidation of Olefins with Palladium Chloride Catalysts. Angew. Chem., Int. Ed. 1962, 1, 80–88. 10.1002/anie.196200801. - DOI
- Hu M.; Wu W.; Jiang H. Palladium-Catalyzed Oxidation Reactions of Alkenes with Green Oxidants. ChemSusChem 2019, 12, 2911–2935. 10.1002/cssc.201900397. - DOI - PubMed
-
- Tsuji J.; Nagashima H.; Nemoto H. A General Synthetic Method for the Preparation of Methyl Ketones from Terminal Olefins: 2-Decanone. Org. Synth. 1984, 62, 9–13. 10.15227/orgsyn.062.0009. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

