This is a preprint.
Evaluation of plasma p-tau217 for detecting amyloid pathology in a diverse and heterogeneous community-based cohort
- PMID: 39974029
- PMCID: PMC11838994
- DOI: 10.1101/2025.01.20.25320851
Evaluation of plasma p-tau217 for detecting amyloid pathology in a diverse and heterogeneous community-based cohort
Update in
-
Evaluation of plasma p-tau217 for detecting amyloid pathology in a heterogeneous community-based cohort.Alzheimers Dement. 2025 Jul;21(7):e70426. doi: 10.1002/alz.70426. Alzheimers Dement. 2025. PMID: 40613474 Free PMC article.
Abstract
Introduction: Studies suggest excellent performance of plasma p-tau217 for detecting amyloid pathology, though studies in more diverse populations are needed to validate previously determined cutpoints.
Methods: Plasma p-tau217 utility for detecting amyloid pathology (Aβ) via amyloid PET (n=598) and/or cerebrospinal fluid (CSF; n=154) was assessed in a heterogeneous, community-based cohort in the Wake Forest Alzheimer's Disease Research Center (WFADRC). Participants (n=598) were 21% Black; 313 cognitive unimpaired (CU), 214 mild cognitive impairment (MCI), and 64 dementia (DEM); 49% prediabetic, 44% hypertensive; 29% overweight/obese; and 64% with mild-to-moderate kidney disease. Gaussian-mixture models, logistic regression, and receiver operating curve analyses were performed.
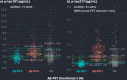
Results: Plasma p-tau217 was associated with elevated Aβ deposition and accurately classified Aβ-positive participants (PET: AUC: 94%-97%, cutpoint≥.338 pg/mL; CSF: AUC = .84, cutpoint≥.307 pg/mL).
Discussion: Plasma p-tau217 is an accurate indicator of amyloid pathology in a heterogeneous cohort, and superior to other plasma biomarkers assessed. Longitudinal analyses assessing impact of comorbidities on p-tau217 utility for disease progression are underway.
Keywords: Biomarkers; Dementia; PET; Plasma; Risk.
Conflict of interest statement
Conflict of Interest Statement Drs. Rudolph, Sutphen, Register, Rundle, Hughes, Solingapuram Sai, and Whitlow, have no conflicts of interest to disclose. Drs. Bateman and Lockhart receive funding from the Alzheimer’s Association. Dr. Bateman has also received honoraria from Efficient CME, PeerView CME, and Novo Nordisck in the last two years. Dr. Craft reports disclosures for vTv Therapeutics, T3D Therapeutics, Cyclerion Inc., and Cognito Inc. Dr. Mielke consults for or serves on advisory boards for Biogen, Eisai, Lilly, Merck, Roche, and Siemens Healthineers.
Figures

References
-
- Rudolph MD, Sutphen CL, Register TC, Whitlow CT, Solingapuram Sai KK, Hughes TM, et al. Associations among plasma, MRI, and amyloid PET biomarkers of Alzheimer’s disease and related dementias and the impact of health-related comorbidities in a community-dwelling cohort. Alzheimer’s & Dementia 2024;20:4159. 10.1002/ALZ.13835. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
