This is a preprint.
Spectral normative modeling of brain structure
- PMID: 39974093
- PMCID: PMC11838943
- DOI: 10.1101/2025.01.16.25320639
Spectral normative modeling of brain structure
Abstract
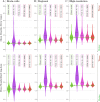
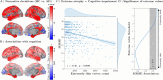
Normative modeling in neuroscience aims to characterize interindividual variation in brain phenotypes and thus establish reference ranges, or brain charts, against which individual brains can be compared. Normative models are typically limited to coarse spatial scales due to computational constraints, limiting their spatial specificity. They additionally depend on fixed regions from fixed parcellation atlases, restricting their adaptability to alternative parcellation schemes. To overcome these key limitations, we propose spectral normative modeling (SNM), which leverages brain eigenmodes for efficient spatial reconstruction to generate normative ranges for arbitrary new regions of interest. Benchmarking against conventional counterparts, SNM achieves a 98.3% speedup in computing accurate normative ranges across spatial scales, from millimeters to the whole brain. We demonstrate its utility by elucidating high-resolution individual cortical atrophy patterns and characterizing the heterogeneous nature of neurodegeneration in Alzheimer's disease. SNM lays the groundwork for a new generation of spatially precise brain charts, offering substantial potential to drive advances in individualized precision medicine.
Keywords: Brain Charts; Brain Eigenmodes; Graph Signal Processing; Normative Modeling.
Figures




References
-
- Dinga R. et al. Normative modeling of neuroimaging data using generalized additive models of location scale and shape. bioRxiv. (2021).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
