This is a preprint.
Distinct clinical phenotypes and their neuroanatomic correlates in chronic traumatic brain injury
- PMID: 39974133
- PMCID: PMC11838966
- DOI: 10.1101/2025.01.27.25321200
Distinct clinical phenotypes and their neuroanatomic correlates in chronic traumatic brain injury
Update in
-
Distinct clinical phenotypes and their neuroanatomic correlates in chronic traumatic brain injury.Brain Commun. 2025 Jun 6;7(3):fcaf216. doi: 10.1093/braincomms/fcaf216. eCollection 2025. Brain Commun. 2025. PMID: 40574978 Free PMC article.
Abstract
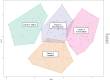
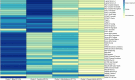
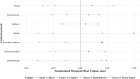
Accumulating evidence of heterogeneous long-term outcomes after traumatic brain injury (TBI) has challenged longstanding approaches to TBI outcome classification that are largely based on global functioning. A lack of studies with clinical and biomarker data from individuals living with chronic (>1 year post-injury) TBI has precluded refinement of long-term outcome classification ontology. Multimodal data in well-characterized TBI cohorts is required to understand the clinical phenotypes and biological underpinnings of persistent symptoms in the chronic phase of TBI. The present cross-sectional study leveraged data from 281 participants with chronic complicated mild-to-severe TBI in the Late Effects of Traumatic Brain Injury (LETBI) Study. Our primary objective was to develop and validate clinical phenotypes using data from 41 TBI measures spanning a comprehensive cognitive battery, motor testing, and assessments of mood, health, and functioning. We performed a 70/30% split of training (n=195) and validation (n=86) datasets and performed principal components analysis to reduce the dimensionality of data. We used Hierarchical Cluster Analysis on Principal Components with k-means consolidation to identify clusters, or phenotypes, with shared clinical features. Our secondary objective was to investigate differences in brain volume in seven cortical networks across clinical phenotypes in the subset of 168 participants with brain MRI data. We performed multivariable linear regression models adjusted for age, age-squared, sex, scanner, injury chronicity, injury severity, and training/validation set. In the training/validation sets, we observed four phenotypes: 1) mixed cognitive and mood/behavioral deficits (11.8%; 15.1% in the training and validation set, respectively); 2) predominant cognitive deficits (20.5%; 23.3%); 3) predominant mood/behavioral deficits (27.7%; 22.1%); and 4) few deficits across domains (40%; 39.5%). The predominant cognitive deficit phenotype had lower cortical volumes in executive control, dorsal attention, limbic, default mode, and visual networks, relative to the phenotype with few deficits. The predominant mood/behavioral deficit phenotype had lower volumes in dorsal attention, limbic, and visual networks, compared to the phenotype with few deficits. Contrary to expectation, we did not detect differences in network-specific volumes between the phenotypes with mixed deficits versus few deficits. We identified four clinical phenotypes and their neuroanatomic correlates in a well-characterized cohort of individuals with chronic TBI. TBI phenotypes defined by symptom clusters, as opposed to global functioning, could inform clinical trial stratification and treatment selection. Individuals with predominant cognitive and mood/behavioral deficits had reduced cortical volumes in specific cortical networks, providing insights into sensitive, though not specific, candidate imaging biomarkers of clinical symptom phenotypes after chronic TBI and potential targets for intervention.
Keywords: Machine Learning; Neuroimaging; Phenotyping; Traumatic brain injury.
Figures


References
-
- Covington NV, Duff MC. Heterogeneity is a hallmark of traumatic brain injury, not a limitation: a new perspective on study design in rehabilitation research. American journal of speech-language pathology. 2021;30(2S):974–985. - PubMed
-
- Dams-O’Connor K, Juengst SB, Bogner J, et al. Traumatic brain injury as a chronic disease: insights from the United States traumatic brain injury model systems research program. The Lancet Neurology. 2023;22(6):517–528. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
