This is a preprint.
The Association of the Microbiome with Melanoma Tumor Response to Immune Checkpoint Inhibitor Treatment and Immune-Related Adverse Events (NCT05102773)
- PMID: 39974142
- PMCID: PMC11838642
- DOI: 10.1101/2025.01.30.25321413
The Association of the Microbiome with Melanoma Tumor Response to Immune Checkpoint Inhibitor Treatment and Immune-Related Adverse Events (NCT05102773)
Abstract
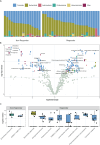
Improved understanding of the factors that underlie immune checkpoint inhibitor (ICI) response and toxicity are needed as only half of patients with metastatic melanoma respond, and 10-40% experience immune-related adverse events (irAEs). Modifying the gut microbiome could positively affect response to ICIs and reduce toxicities. Here, we sought to determine if the pre-treatment gut microbiome predicts ICI response or toxicity in the setting of metastatic melanoma. Melanoma patients (n=88) over 18 years of age, planning to receive ICI therapy enrolled in a prospective observational cohort study at The Ohio State University Comprehensive Cancer Center Skin Cancer Clinic. Patients taking corticosteroids for indications other than adrenal physiologic replacement were excluded. Stools were collected at baseline, within 10 days of an irAE as determined by CTCAE v 5.0 criteria, and at 12 weeks. ICI response and progression-free survival (PFS) were evaluated q12 weeks using Response Evaluation Criteria in Solid Tumors (RECIST v1.1). Metagenomic whole-genome shotgun sequencing of the microbiome was classified using MetaPhlAn4/HUMAnN3 and differential abundance analyzed with ANCOM-BC2. Of the 88 patients enrolled, 41 had metastatic disease and complete data. There were 25 participants classified as responders, defined as having complete response or partial response according to RECIST criteria, or stable disease with 6-month PFS. Grade ≥ 1 irAEs were observed in 15/41 participants. The abundance of Intestinimonas butyriciproducens (q-value = 0.002) and Longicatena caecimuris (q-value = 0.003) were enriched in responders, Tenericutes (q-value= 0.001) and Lachnospira sp. NSJ 43 (q-value =0.002) in non-responders. Blautia luti, as well as several other Lachnospiraceae, were associated with response and no irAE (response q-value = 0.02, no irAE q-value = 0.02). The association of response to ICIs with several taxa in the family Lachnospiraceae, a prevalent microbial family in the gut, is consistent with prior research, which has found that this family may influence treatment outcomes through various mechanisms, such as immune regulation, metabolism, and pathogen exclusion. While no statistical relationship was observed between response and irAEs in this cohort, the microbes associated with both could serve as biomarkers. Future studies to assign causal roles for (specific microbes) in response and toxicity could identify mechanisms to improve patient outcomes.
Keywords: immune-related adverse events; immunotherapy; microbiome.
Figures


References
-
- Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, et al. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia. 2017;19:848–55. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
