Sex-based differences in the long-term fate of hippocampal neurons born after a traumatic brain injury
- PMID: 39974293
- PMCID: PMC11836013
- DOI: 10.3389/fnbeh.2025.1523969
Sex-based differences in the long-term fate of hippocampal neurons born after a traumatic brain injury
Abstract
Introduction: Moderate-to-severe traumatic brain injury (TBI) results in an early loss of immature hippocampal granule cells and the activation of typically quiescent neural stem cells (NSCs) in the dentate gyrus. Activation of NSCs leads to a robust increase in proliferation and generation of neural progenitor cells (NPCs), supporting restoration of the immature neuron population of over a period of 1-2 weeks. However, it is unclear if neurons born early after injury develop normally, survive long-term and functionally integrate into the hippocampal network. Although adult hippocampal neurogenesis is regulated in a sex-dependent manner, the majority of pre-clinical TBI studies lack the inclusion of both sexes. The goal of this study was to examine sex differences in hippocampal neurogenesis in response to a moderate controlled cortical impact brain injury.
Methods: In-vivo labeling of NPCs and tracking of their morphological development into a granule cell was achieved using an inducible Cre recombinase driven by the Ascl1 promoter in a CAG-floxStopTom reporter mouse. Ascl1 is a basic helix-loop-helix transcription factor transiently expressed in NPCs and activated NSCs in the dentate gyrus of the adult mammalian brain. To specifically label NPCs born acutely after TBI, tamoxifen was delivered to mice on days 2 and 3 postinjury. Mice survived to 6 weeks after TBI to allow for full neuronal maturation of tdTomato-labeled NPCs.
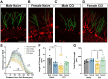
Results: At 6 weeks postinjury, numbers of tdTomato-positive granule cells were significantly reduced in the ipsilateral hippocampus of brain-injured mice compared to controls, with a more pronounced decrease in males. Further, posttrauma-born neurons in males, but not females, exhibited impaired dendritic development. Neurons born after injury extended axons which formed synaptic terminals within the CA3 region. Numbers of mossy fiber boutons were significantly decreased in injured males compared to naïve males or to injured females. Potential forms of plasticity were observed in brain-injured females, including increased neurogenesis in the contralateral hippocampus and increased mossy fiber bouton volume. Together these data suggest a neurogenic advantage in females after injury.
Discussion: This study is the first to report sex differences in posttraumatic hippocampal neurogenesis and to demonstrate modification of synaptic terminals formed by neurons born after TBI.
Keywords: dendrite morphology; dentate gyrus; hippocampus; mossy fiber boutons; neurogenesis; sex differences; synapses; traumatic brain injury.
Copyright © 2025 Downing, Glover, Gebhardt, Thompson and Saatman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

