Fingolimod-Associated Central Serous Retinopathy Presenting Eight Years after Treatment Initiation: a Case Report
- PMID: 39974447
- PMCID: PMC11834850
- DOI: 10.26574/maedica.2024.19.4.869
Fingolimod-Associated Central Serous Retinopathy Presenting Eight Years after Treatment Initiation: a Case Report
Abstract
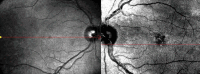
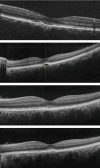
Fingolimod is an efficacious treatment option in the management of multiple sclerosis, while variable ocular adverse effects have been associated with its use. Herein, we present a case with development of central serous retinopathy (CSR) in the long-term after commencement of fingolimod treatment. A 53-year-old female with relapsing-remitting multiple sclerosis presented with central vision metamorphopsia in the left eye. Medical history revealed that the patient had been on treatment with fingolimod for eight years. Ophthalmological examination revealed retinal findings suggestive of CSR changes. Given the necessity to continue fingolimod treatment, the patient commenced topical nonsteroidal anti-inflammatory (nepafenac) medication for the retinal condition. There was a prompt and successful resolution of fluid following treatment; however, a subsequent presumed ocular surface disease urged the discontinuation of anti-inflammatory drops. Four weeks later, the patient presented with recurrence of fluid in the subRPE level. Thereafter, fingolimod treatment was switched to dimethyl fumarate. The patient's condition remained clinically stable without recurrence of fluid during a three-month follow-up period. Our case provides insight into the fingolimod associated retinal findings manifesting as CSR changes, indicating that ocular complications may occur even in the long-term, while discontinuation of treatment may lead to reversal of structural findings and maintain functional outcomes.
Figures


References
-
- Kappos L, Radue E, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. - PubMed
-
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. - PubMed
-
- Volpi C, Orabona C, Macchiarulo A, et al. Preclinical discovery and development of fingolimod for the treatment of multiple sclerosis. Expert Opin Drug Discov. 2019;14:1199–1212. - PubMed
-
- Zarbin MA, Jampol LM, Jager RD. Ophthalmic evaluations in clinical studies of fingolimod (FTY720) in multiple sclerosis. Ophthalmology. 2013;120:1432–1439. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
