COVID-19 vaccination and birth outcomes of 186,990 women vaccinated before pregnancy: an England-wide cohort study
- PMID: 39974773
- PMCID: PMC11838104
- DOI: 10.1016/j.lanepe.2024.101025
COVID-19 vaccination and birth outcomes of 186,990 women vaccinated before pregnancy: an England-wide cohort study
Abstract
Background: COVID-19 vaccination in pregnancy is recommended by the World Health Organisation as effective and safe. However, there remains a lack of robust evidence to inform vaccination choices for women of childbearing potential in relation to their future pregnancies. Here we investigated the association between starting a course of COVID-19 vaccination before pregnancy and birth outcomes.
Methods: We analysed England-wide linked electronic health records for all pregnancies reaching at least 24 weeks gestation between 25th May 2021 and 28th October 2022. We estimated incidence rates and hazard ratios for birth and pregnancy outcomes by pre-pregnancy COVID-19 vaccination status.
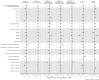
Findings: Based on 186,990 women, compared to starting a pregnancy unvaccinated, receiving COVID-19 vaccination within 12 months before pregnancy was associated with lower risks of very and extremely preterm birth and small-for-gestational age in term babies for any vaccine type (adjusted hazard ratio and 95% confidence interval: 0.74 [0.63, 0.88] and 0.94 [0.88, 1.00], respectively), and lower stillbirth risk in those receiving an mRNA vaccine (0.72 [0.52, 1.00]). Incidence of venous thromboembolism during pregnancy was higher amongst women receiving a viral-vector, but not an mRNA vaccine (1.54 [1.10, 2.16] and 1.02 [0.70, 1.50], respectively). Results were generally consistent for different dose regimens and across sensitivity analyses.
Interpretation: We found evidence that pregnancies starting within 12 months from a first COVID-19 vaccination, compared to those in unvaccinated women, experienced fewer adverse birth outcomes, overall or in selected subgroups of the general population, accounting for potential confounders. An mRNA vaccine should be preferred to a viral-vector vaccine, to minimise safety issues, but where the latter is the only choice, it is still to be preferred to starting a pregnancy unvaccinated. The venous thromboembolism risk of the viral-vector vaccine was substantially lower compared to that attributable to SARS-CoV-2 infection in pregnancy or to commonly used medications such as hormone replacement therapy and oral contraceptives in the non-pregnant population.
Funding: UK National Institute for Health and Care Research (NIHR), UKRIMedical Research Council, UK Research and Innovation, The Alan Turing Institute, Health Data Research UK, the Department of Health and Social Care.
Keywords: COVID-19 vaccination; Electronic health records; Pregnancy; Preterm; Stillbirth; Venous thromboembolism.
© 2024 The Authors.
Conflict of interest statement
CT has received funding paid to University College London from GlaxoSmithKline (GSK), outside the scope of the submitted work. The other Authors declare no conflict of interest.
Figures

References
-
- World Health Organisation COVID-19 vaccines. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19... [cited 2024 Jan 22]. Available from:
-
- Health Security Agency COVID-19 vaccination: a guide on pregnancy and breastfeeding. HM Government. https://www.gov.uk/government/publications/covid-19-vaccination-women-of... [cited 2024 Apr 1]. Available from:
LinkOut - more resources
Full Text Sources
Miscellaneous

