Wearable penile devices: the TechRing
- PMID: 39974806
- PMCID: PMC11833538
- DOI: 10.21037/tau-24-548
Wearable penile devices: the TechRing
Abstract
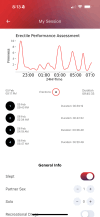
The integration of wearable technology into healthcare has transformed patient monitoring, particularly in urology. Wearable penile devices (WPDs), such as the FirmTech TechRing, offer real-time data on erectile function, providing critical insights into nocturnal penile tumescence (NPT) patterns that can indicate underlying health issues. This piece highlights the potential of the FirmTech TechRing, a WPD, to advance erectile function monitoring by providing real-time data on NPT and intra-coital activity. It explores its promise in detecting early dysfunction, assessing cardiovascular risk, and enhancing proactive health management. By measuring parameters like firmness, girth expansion, and the frequency of erections, the TechRing enables proactive monitoring of erectile health, potentially predicting conditions like erectile dysfunction (ED) and cardiovascular disease earlier than traditional methods. In comparison to conventional diagnostic tools like RigiScan, the TechRing is a cost-effective alternative aimed at consumers, facilitating personal health tracking without the need for clinical environments. Future advancements may include enhanced sensors for assessing blood flow dynamics, measuring penile diameter, and detecting vascular conditions, potentially leading to improved diagnostic accuracy and personalized treatment plans. The TechRing exemplifies the potential of WPD to significantly enrich sexual medicine, providing clinicians and patients with valuable data to inform health decisions, ultimately enhancing patient care and quality of life. The convergence of these technologies marks a promising evolution in erectile health management, empowering users and healthcare professionals alike with actionable insights for informed interventions.
Keywords: Nocturnal penile tumescence (NPT); penile erection; penile tumescence; wearable device data; wearable devices.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-24-548/coif). R.D. is an employee of FirmTech Inc. M.K. is a consultant for Abbvie, Boston Scientific, Acerus, Coloplast, Endo, Tolmar, Marius, Petros, Halozyme, the President of SMSNA, and an investor on Sprout and Vault. J.P.M. is an advisor for FirmTech Inc. The other authors have no conflicts of interest to declare.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources