This is a preprint.
Molecular determinants of RNase MRP specificity and function
- PMID: 39974906
- PMCID: PMC11838342
- DOI: 10.1101/2025.01.28.635360
Molecular determinants of RNase MRP specificity and function
Abstract
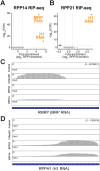
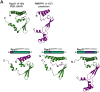
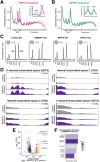
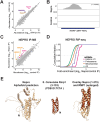
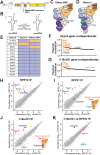
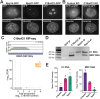
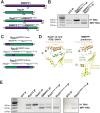
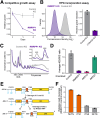
RNase MRP and RNase P are evolutionarily related complexes that facilitate rRNA and tRNA biogenesis, respectively. The two enzymes share nearly all protein subunits and have evolutionarily related catalytic RNAs. Notably, RNase P includes a unique subunit, Rpp21, whereas no RNase MRP-specific proteins have been found in humans, limiting molecular analyses of RNase MRP function. Here, we identify the RNase MRP-specific protein, C18orf21/RMRPP1. RMRPP1 and Rpp21 display significant structural homology, but we identify specific regions that drive interactions with their respective complexes. Additionally, we reveal that RNase MRP is required for 40S, but not 60S, ribosome biogenesis uncovering an alternative pathway for ribosome assembly. Finally, we identify Nepro as an essential rRNA processing factor that associates with the RNase MRP complex. Together, our findings elucidate the molecular determinants of RNase MRP function and underscore its critical role in ribosome biogenesis.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
