This is a preprint.
Clostridioides difficile major toxins remodel the intestinal epithelia, affecting spore adherence/internalization into intestinal tissue and their association with gut vitronectin
- PMID: 39974910
- PMCID: PMC11838273
- DOI: 10.1101/2025.01.29.635439
Clostridioides difficile major toxins remodel the intestinal epithelia, affecting spore adherence/internalization into intestinal tissue and their association with gut vitronectin
Abstract
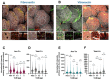
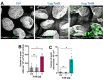
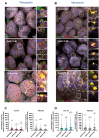
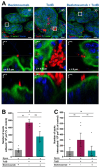
The most common cause of healthcare-associated diarrhea and colitis in the U.S., is Clostridioides difficile, a spore-forming pathogen. Two toxins, TcdA and TcdB, are major virulence factors essential for disease manifestations, while C. difficile spores are essential for disease transmission and recurrence. Both toxins cause major damage to the epithelial barrier, trigger massive inflammation, and reshape the microbiome and metabolic composition, facilitating C. difficile colonization. C. difficile spores, essential for transmission and recurrence of the disease, persist adhered and internalized in the intestinal epithelia. Studies have suggested that toxin-neutralization in combination with antibiotic during CDI treatment in humans significantly reduces disease recurrence, suggesting a link between toxin-mediated damage and spore persistence. Here, we show that TcdA/TcdB-intoxication of intestinal epithelial Caco-2 cells leads to remodeling of accessible levels of fibronectin (Fn) and vitronectin (Vn) and their cognate alpha-integrin subunits. While TcdB-intoxication of intestinal tissue had no impact in accessible levels of Fn and Vn, but significantly increased levels of intracellular Vn. We observed that Fn and Vn released to the supernatant readily bind to C. difficile spores in vitro, while TcdB-intoxication of intestinal tissue led to increased association of C. difficile spores with gut Vn. Toxin-intoxication of the intestinal tissue also contributes to increased adherence and internalization of C. difficile spores. However, TcdB-intoxicated ligated loops infected of mice treated with Bezlotoxumanb (monoclonal anti-TcdB antibodies) did not prevent TcdB-mediated increased spore adherence and internalization into intestinal tissue. This study highlights the importance of studying the impact of C. difficile toxins of host tissues has in C. difficile interaction with host surfaces that may contribute to increased persistence and disease recurrence.
Figures




References
-
- Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–34. Epub 2015/02/26. doi: 10.1056/NEJMoa1408913. - DOI - PMC - PubMed
-
- McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Shaklee Sammons J, Sandora TJ, Wilcox MH. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987–94. doi: 10.1093/cid/ciy149. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
