This is a preprint.
Fsr quorum sensing system restricts biofilm growth and activates inflammation in enterococcal infective endocarditis
- PMID: 39974957
- PMCID: PMC11839028
- DOI: 10.1101/2025.02.07.636843
Fsr quorum sensing system restricts biofilm growth and activates inflammation in enterococcal infective endocarditis
Abstract
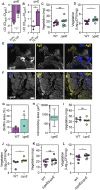
Infective endocarditis (IE) is a life-threatening biofilm-associated infection, yet the factors driving biofilm formation remain poorly understood. Here, we identified the Fsr quorum sensing (QS) system of Enterococcus faecalis as a potent negative regulator of IE pathogenesis. Using microfluidic and in vivo models, we show that Fsr is induced in late IE when bacteria become shielded from blood flow. Deleting Fsr altered biofilm metabolism and promoted robust biofilm growth and gentamicin tolerance in vivo. Furthermore, Fsr inactivation attenuated inflammation by disrupting IL-1β cleavage and activation via the Fsr-regulated gelatinase (gelE), allowing biofilm to grow unchecked by the immune system. Consistent with our pre-clinical findings, analysis of two IE patient cohorts linked naturally occurring Fsr-deficient E. faecalis to prolonged bacteremia. Overall, our findings provide insights into the role of QS in biofilm growth, persistence, and immune evasion in enterococcal IE.
Keywords: Enterococcus faecalis; Fsr; IL-1β; biofilm; gelatinase; immune evasion; infective endocarditis; quorum sensing.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
