This is a preprint.
Multiplexed CRISPRi Reveals a Transcriptional Switch Between KLF Activators and Repressors in the Maturing Neocortex
- PMID: 39975013
- PMCID: PMC11839100
- DOI: 10.1101/2025.02.07.636951
Multiplexed CRISPRi Reveals a Transcriptional Switch Between KLF Activators and Repressors in the Maturing Neocortex
Abstract
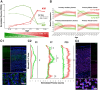
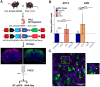
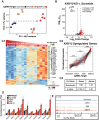
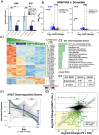
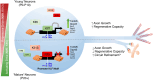
A critical phase of mammalian brain development takes place after birth. Neurons of the mouse neocortex undergo dramatic changes in their morphology, physiology, and synaptic connections during the first postnatal month, while properties of immature neurons, such as the capacity for robust axon outgrowth, are lost. The genetic and epigenetic programs controlling prenatal development are well studied, but our understanding of the transcriptional mechanisms that regulate postnatal neuronal maturation is comparatively lacking. By integrating chromatin accessibility and gene expression data from two subtypes of neocortical pyramidal neurons in the neonatal and maturing brain, we predicted a role for the Krüppel-Like Factor (KLF) family of Transcription Factors in the developmental regulation of neonatally expressed genes. Using a multiplexed CRISPR Interference (CRISPRi) knockdown strategy, we found that a shift in expression from KLF activators (Klf6, Klf7) to repressors (Klf9, Klf13) during early postnatal development functions as a transcriptional 'switch' to first activate, then repress a set of shared targets with cytoskeletal functions including Tubb2b and Dpysl3. We demonstrate that this switch is buffered by redundancy between KLF paralogs, which our multiplexed CRISPRi strategy is equipped to overcome and study. Our results indicate that competition between activators and repressors within the KLF family regulates a conserved component of the postnatal maturation program that may underlie the loss of intrinsic axon growth in maturing neurons. This could facilitate the transition from axon growth to synaptic refinement required to stabilize mature circuits.
Figures



Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Peripuberty Is a Sensitive Period for Prefrontal Parvalbumin Interneuron Activity to Impact Adult Cognitive Flexibility.Dev Neurosci. 2025;47(2):127-138. doi: 10.1159/000539584. Epub 2024 Jun 3. Dev Neurosci. 2025. PMID: 38830346 Free PMC article.
-
A transcriptional constraint mechanism limits the homeostatic response to activity deprivation in mammalian neocortex.Elife. 2023 Feb 7;12:e74899. doi: 10.7554/eLife.74899. Elife. 2023. PMID: 36749029 Free PMC article.
-
Telephone support for women during pregnancy and the first six weeks postpartum.Cochrane Database Syst Rev. 2013 Jul 18;2013(7):CD009338. doi: 10.1002/14651858.CD009338.pub2. Cochrane Database Syst Rev. 2013. PMID: 23881662 Free PMC article.
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
References
-
- Apara A., Galvao J., Wang Y., Blackmore M., Trillo A., Iwao K., Brown D. P., Fernandes K. A., Huang A., Nguyen T., Ashouri M., Zhang X., Shaw P. X., Kunzevitzky N. J., Moore D. L., Libby R. T., & Goldberg J. L. (2017). KLF9 and JNK3 Interact to Suppress Axon Regeneration in the Adult CNS. Journal of Neuroscience, 37(40), 9632–9644. 10.1523/JNEUROSCI.0643-16.2017 - DOI - PMC - PubMed
-
- Avci H. X., Lebrun C., Wehrlé R., Doulazmi M., Chatonnet F., Morel M.-P., Ema M., Vodjdani G., Sotelo C., Flamant F., & Dusart I. (2012). Thyroid hormone triggers the developmental loss of axonal regenerative capacity via thyroid hormone receptor α1 and Krüppel-Like factor 9 in Purkinje cells. Proceedings of the National Academy of Sciences of the United States of America, 109(35), 14206–14211. 10.1073/pnas.1119853109 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
