This is a preprint.
Attenuated adenosine mediated immune-dampening increases natural killer cell activity in early age-related macular degeneration
- PMID: 39975064
- PMCID: PMC11838234
- DOI: 10.1101/2025.01.22.634301
Attenuated adenosine mediated immune-dampening increases natural killer cell activity in early age-related macular degeneration
Abstract
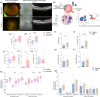
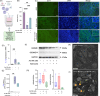
Non-exudative age-related macular degeneration (AMD) involves retinal pigment epithelium (RPE) dysfunction and has been linked to altered intraocular immunity. Our investigation focuses on immune cell subsets and inflammation-associated factors in the eyes with early and intermediate AMD. We observed elevated levels of activated natural killer (NK) cells and interferon-γ, concurrent with reduced myeloid-derived suppressor cells (MDSCs) and adenosine in AMD eyes. Aqueous humor from AMD patients had diminished ability to dampen NK cell activation, an effect rescued by adenosine supplementation. The Cryba1 cKO mouse model recapitulated these immune alterations, and single-cell RNA-sequencing identified NK cell-related genes and NK cell-RPE interactions. Co-culture of activated NK cells with RPE cells induced barrier dysfunction and Gasdermin-E driven pyroptosis providing a functional link relevant to AMD. These findings suggest a double-hit model where elevated immune activation and loss of immune dampening mechanisms drive AMD progression. Resetting the intraocular immune balance may be a promising therapeutic strategy for managing early and intermediate AMD.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
