This is a preprint.
Heterochronous laminar maturation in the human prefrontal cortex
- PMID: 39975178
- PMCID: PMC11838308
- DOI: 10.1101/2025.01.30.635751
Heterochronous laminar maturation in the human prefrontal cortex
Abstract
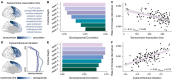
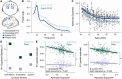
The human prefrontal cortex (PFC) exhibits markedly protracted developmental plasticity, yet whether reductions in plasticity occur synchronously across prefrontal cortical layers is unclear. Animal studies have shown that intracortical myelin consolidates neural circuits to close periods of plasticity. Here, we use quantitative myelin imaging collected from youth (ages 10-32 years) at ultra-high field (7T) to investigate whether deep and superficial PFC layers exhibit different timeframes of plasticity. We find that myelin matures along a deep-to-superficial axis in the PFC; this axis of maturational timing is expressed to a different extent in cytoarchitecturally distinct regions along the frontal cortical hierarchy. By integrating myelin mapping with electroencephalogram and cognitive phenotyping, we provide evidence that deep and superficial prefrontal myelin dissociably impact timescales of neural activity, task learning rates, and cognitive processing speed. Heterochronous maturation across deep and superficial layers is an underrecognized mechanism through which association cortex balances cognitively-relevant increases in circuit stability and efficiency with extended neuroplasticity.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures





Similar articles
-
Peripuberty Is a Sensitive Period for Prefrontal Parvalbumin Interneuron Activity to Impact Adult Cognitive Flexibility.Dev Neurosci. 2025;47(2):127-138. doi: 10.1159/000539584. Epub 2024 Jun 3. Dev Neurosci. 2025. PMID: 38830346 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Incentives for preventing smoking in children and adolescents.Cochrane Database Syst Rev. 2012 Oct 17;10:CD008645. doi: 10.1002/14651858.CD008645.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Jun 06;6:CD008645. doi: 10.1002/14651858.CD008645.pub3. PMID: 23076949 Updated.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Reading aids for adults with low vision.Cochrane Database Syst Rev. 2018 Apr 17;4(4):CD003303. doi: 10.1002/14651858.CD003303.pub4. Cochrane Database Syst Rev. 2018. PMID: 29664159 Free PMC article.
References
-
- Buckner R. L. & Krienen F. M. The evolution of distributed association networks in the human brain. Trends Cogn Sci 17, 648–665 (2013). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
