This is a preprint.
Strategies to decipher neuron identity from extracellular recordings in the cerebellum of behaving non-human primates
- PMID: 39975199
- PMCID: PMC11838295
- DOI: 10.1101/2025.01.29.634860
Strategies to decipher neuron identity from extracellular recordings in the cerebellum of behaving non-human primates
Update in
-
Strategies to Decipher Neuron Identity from Extracellular Recordings in Behaving Nonhuman Primates.J Neurosci. 2025 Aug 6;45(32):e0230252025. doi: 10.1523/JNEUROSCI.0230-25.2025. J Neurosci. 2025. PMID: 40628525
Abstract
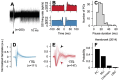
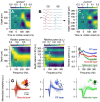
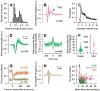
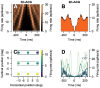
Identification of neuron type is critical to understand computation in neural circuits through extracellular recordings in awake, behaving animal subjects. Yet, modern recording probes have limited power to resolve neuron type. Here, we leverage the well-characterized architecture of the cerebellar circuit to perform expert identification of neuron type from extracellular recordings in behaving non-human primates. Using deep-learning classifiers we evaluate the information contained in readily accessible extracellular features for neuron identification. Waveform, discharge statistics, anatomical layer, and functional interactions each can inform neuron labels for a sizable fraction of cerebellar units. Together, as inputs to a deep-learning classifier, the features perform even better. Our tools and methodologies, validated during smooth pursuit eye movements in the cerebellar floccular complex of awake behaving monkeys, can guide expert identification of neuron type during cerebellar-dependent tasks in behaving animals across species. They lay the groundwork for characterization of information processing in the cerebellar cortex.
Keywords: Golgi cell; Purkinje cell; cell type; classification; molecular layer interneuron; mossy fiber; unipolar brush cell.
Conflict of interest statement
Conflicts of interest The authors declare no conflicts of interest.
Figures




Similar articles
-
Strategies to Decipher Neuron Identity from Extracellular Recordings in Behaving Nonhuman Primates.J Neurosci. 2025 Aug 6;45(32):e0230252025. doi: 10.1523/JNEUROSCI.0230-25.2025. J Neurosci. 2025. PMID: 40628525
-
A deep learning strategy to identify cell types across species from high-density extracellular recordings.Cell. 2025 Apr 17;188(8):2218-2234.e22. doi: 10.1016/j.cell.2025.01.041. Epub 2025 Feb 28. Cell. 2025. PMID: 40023155
-
Neural circuit mechanisms to transform cerebellar population dynamics for motor control in monkeys.bioRxiv [Preprint]. 2025 Feb 22:2025.02.21.639459. doi: 10.1101/2025.02.21.639459. bioRxiv. 2025. PMID: 40027752 Free PMC article. Preprint.
-
Automated monitoring compared to standard care for the early detection of sepsis in critically ill patients.Cochrane Database Syst Rev. 2018 Jun 25;6(6):CD012404. doi: 10.1002/14651858.CD012404.pub2. Cochrane Database Syst Rev. 2018. PMID: 29938790 Free PMC article.
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
References
-
- Lisberger S. G. Visual Guidance of Smooth Pursuit Eye Movements. Annu. Rev. Vis. Sci. 1, 447–468 (2015). - PubMed
-
- Ramón y Cajal S. Histologie Du Système Nerveux de l’homme & Des Vertébrés. (Maloine, Paris, 1909). doi: 10.5962/bhl.title.48637. - DOI
-
- Fishell G. & Heintz N. The Neuron Identity Problem: Form Meets Function. Neuron 80, 602–612 (2013). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
