This is a preprint.
Bayesian Inference of Binding Kinetics from Fluorescence Time Series
- PMID: 39975252
- PMCID: PMC11838460
- DOI: 10.1101/2025.02.03.636267
Bayesian Inference of Binding Kinetics from Fluorescence Time Series
Update in
-
Bayesian Inference of Binding Kinetics from Fluorescence Time Series.J Phys Chem B. 2025 May 15;129(19):4670-4681. doi: 10.1021/acs.jpcb.5c01180. Epub 2025 May 7. J Phys Chem B. 2025. PMID: 40331818
Abstract
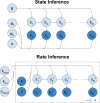
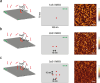
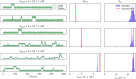
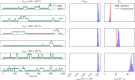
The study of binding kinetics via the analysis of fluorescence time traces is often confounded by measurement noise and photophysics. Although photoblinking can be mitigated by using labels less likely to photoswitch, photobleaching generally cannot be eliminated. Current methods for measuring binding and unbinding rates are therefore limited by concurrent photobleaching events. Here, we propose a method to infer binding and unbinding rates alongside photobleaching rates using fluorescence intensity traces. Our approach is a two-stage process involving analyzing individual regions of interest (ROIs) with a Hidden Markov Model to infer the fluorescence intensity levels of each trace. We then use the inferred intensity level state trajectory from all ROIs to infer kinetic rates. Our method has several advantages, including the ability to analyze noisy traces, account for the presence of photobleaching events, and provide uncertainties associated with the inferred binding kinetics. We demonstrate the effectiveness and reliability of our method through simulations and data from DNA origami binding experiments.
Conflict of interest statement
Competing Interests SP and JSB acknowledge a competing interest with their affiliation with Saguaro Solutions.
Figures

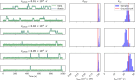
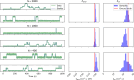
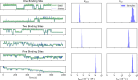
References
-
- Debnath Sibali and Raghavachari Krishnan. “Investigating the Stacking Interactions Responsible for Driving 3D Self-Association of Tricarb Macrocycles”. In: The Journal of Physical Chemistry A 127.39 (2023), pp. 8110–8116. - PubMed
-
- German Helen W, Uyaver Sahin, and Hansmann Ulrich HE. “Self-assembly of phenylalanine-based molecules”. In: The Journal of Physical Chemistry A 119.9 (2014), pp. 1609–1615. - PubMed
-
- Petroski Janet M, Green Travis C, and El-Sayed Mostafa A. “Self-assembly of platinum nanoparticles of various size and shape”. In: The Journal of Physical Chemistry A 105.23 (2001), pp. 5542–5547.
-
- Banerjee Kinshuk, Kolomeisky Anatoly B, and Igoshin Oleg A. “Accuracy of substrate selection by enzymes is controlled by kinetic discrimination”. In: The journal of physical chemistry letters 8.7 (2017), pp. 1552–1556. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
