This is a preprint.
Chronic alcohol consumption enhances the differentiation capacity of hematopoietic stem and progenitor cells into osteoclast precursors
- PMID: 39975302
- PMCID: PMC11839057
- DOI: 10.1101/2025.02.05.636743
Chronic alcohol consumption enhances the differentiation capacity of hematopoietic stem and progenitor cells into osteoclast precursors
Update in
-
Chronic Alcohol Consumption Enhances the Differentiation Capacity of Hematopoietic Stem and Progenitor Cells into Osteoclast Precursors.Am J Pathol. 2025 Jul 17:S0002-9440(25)00244-5. doi: 10.1016/j.ajpath.2025.06.010. Online ahead of print. Am J Pathol. 2025. PMID: 40683559
Abstract
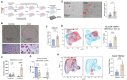
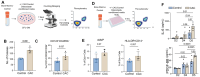
Chronic alcohol consumption (CAC) is associated with an enhanced risk of bone fracture, reduced bone density, and osteoporosis. We have previously shown using a rhesus macaque model of voluntary ethanol consumption that CAC induces functional, transcriptomic, and epigenomic changes in hematopoietic stem and progenitor cells (HSPCs) and their resultant monocytes/macrophages, skewing them towards a hyper-inflammatory response. Here, we extended those studies and investigated alterations in osteoclasts, which, in postnatal life, are differentiated from HSPCs and play a critical role in maintaining bone homeostasis. Analysis using spectral flow cytometry revealed a skewing of HSPCs towards granulocyte-monocyte progenitors (GMPs) with the CAC group that was in concordance with an increased number of colony-forming unit-granulocyte/macrophage (CFU-GM). Additionally, HSPCs from animals in the CAC group incubated with M-CSF and RANKL were more likely to differentiate into osteoclasts, as evidenced by increased Tartrate-Resistant Acid Phosphatase (TRAP) staining and bone resorption activity. Moreover, single-cell RNA sequencing of differentiated HSPCs identified three clusters of osteoclast precursors in the CAC group with enhanced gene expression in pathways associated with cellular response to stimuli, membrane trafficking, and vesicle-mediated transport. Collectively, these data show that CAC-derived hematopoietic progenitor cells exhibit a higher capacity to differentiate into osteoclast precursors. These findings provide critical insights for future research on the mechanisms by which CAC disrupts monopoiesis homeostasis and enhances osteoclast precursors, thereby contributing to reduced bone density.
Keywords: Chronic alcohol consumption; Hematopoietic stem and progenitor cells; Osteoclast; Osteoclast precursors; Osteoclastogenesis; Osteoporosis.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest.
Figures



References
-
- SAMHSA CfBHSaQ. National Survey on Drug Use and Health. 2022. [cited 2024 December 18, 2023]. Available from: https://www.samhsa.gov/data/sites/default/files/reports/rpt42728/NSDUHDe....
-
- Godos J, Giampieri F, Chisari E, Micek A, Paladino N, Forbes-Hernandez TY, Quiles JL, Battino M, La Vignera S, Musumeci G, Grosso G. Alcohol Consumption, Bone Mineral Density, and Risk of Osteoporotic Fractures: A Dose-Response Meta-Analysis. Int J Environ Res Public Health. 2022;19(3). Epub 20220128. doi: 10.3390/ijerph19031515. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
