This is a preprint.
Endothelial Cu Uptake Transporter CTR1 Senses Disturbed Flow to Promote Atherosclerosis through Cuproptosis
- PMID: 39975331
- PMCID: PMC11838200
- DOI: 10.1101/2025.01.27.634587
Endothelial Cu Uptake Transporter CTR1 Senses Disturbed Flow to Promote Atherosclerosis through Cuproptosis
Abstract
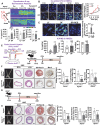
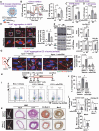
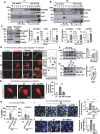
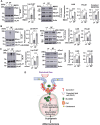
Endothelial cells (ECs) lining blood vessels sense disturbed blood flow (D-flow), which drives mitochondrial dysfunction and atherosclerosis. Copper (Cu) is an essential micronutrient, and its disruption of homeostasis has been implicated in atherosclerosis. Cellular Cu levels are tightly controlled by Cu transport proteins including the Cu importer CTR1. Cuproptosis is a recently discovered form of regulated cell death triggered by mitochondrial Cu accumulation, but its endogenous stimulants and role in atherosclerosis remain unknown. Using EC-specific CTR1-deficient mice and cultured ECs, we show that endothelial CTR1 responds to D-flow by increasing mitochondrial Cu levels through its interaction with the mitochondrial Cu transporter SLC25A3 at caveolae/lipid rafts. This leads to the aggregation of lipoylated mitochondrial proteins, mitochondrial dysfunction, and cuproptosis, thereby exacerbating atherosclerosis. Importantly, mitochondria-targeted Cu-chelating nanoparticles effectively mitigate D-flow-induced cuproptosis and atherosclerosis, highlighting the endothelial CTR1-SLC25A3-mitochondrial Cu axis as a potential therapeutic target.
Conflict of interest statement
Competing interests: Authors declared that they have no competing interests.
Figures

References
-
- Cunningham K. S., Gotlieb A. I., The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest 85, 9–23 (2005). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
