This is a preprint.
Cholesterol-mediated Lysosomal Dysfunction in APOE4 Astrocytes Promotes α-Synuclein Pathology in Human Brain Tissue
- PMID: 39975381
- PMCID: PMC11839026
- DOI: 10.1101/2025.02.09.637107
Cholesterol-mediated Lysosomal Dysfunction in APOE4 Astrocytes Promotes α-Synuclein Pathology in Human Brain Tissue
Abstract
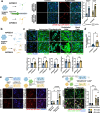
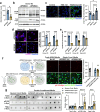
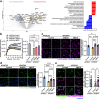
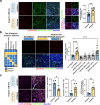
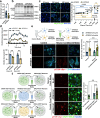
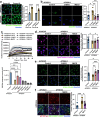
The pathological hallmark of neurodegenerative disease is the aberrant post-translational modification and aggregation of proteins leading to the formation of insoluble protein inclusions. Genetic factors like APOE4 are known to increase the prevalence and severity of tau, amyloid, and α-Synuclein inclusions. However, the human brain is largely inaccessible during this process, limiting our mechanistic understanding. Here, we developed an iPSC-based 3D model that integrates neurons, glia, myelin, and cerebrovascular cells into a functional human brain tissue (miBrain). Like the human brain, we found pathogenic phosphorylation and aggregation of α-Synuclein is increased in the APOE4 miBrain. Combinatorial experiments revealed that lipid-droplet formation in APOE4 astrocytes impairs the degradation of α-synuclein and leads to a pathogenic transformation that seeds neuronal inclusions of α-Synuclein. Collectively, this study establishes a robust model for investigating protein inclusions in human brain tissue and highlights the role of astrocytes and cholesterol in APOE4-mediated pathologies, opening therapeutic opportunities.
Keywords: APOE4; Alzheimer’s Disease; Astrocytes; Cholesterol metabolism; Lewy Body Dementia; Lysosomal dysfunction; Neurodegeneration; iPSC-derived brain model; miBrain; α-Synuclein.
Conflict of interest statement
Declaration of Interests V.K. is a cofounder of and senior advisor to DaCapo Brainscience and Yumanity Therapeutics, companies focused on CNS diseases. L.A.M.L, J.W.B., C.G., and R.B.R. are inventors on patent applications filed by Mount Sinai Innovation Partners on the methods described in this study. A.N. and V.K. are inventors on a patent application filed by Brigham and Women’s Hospital related to the induced inclusion iPSC models.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
