This is a preprint.
Kinase Plasticity in Response to Vandetanib Enhances Sensitivity to Tamoxifen in Estrogen Receptor Positive Breast Cancer
- PMID: 39975402
- PMCID: PMC11838206
- DOI: 10.1101/2024.12.19.629395
Kinase Plasticity in Response to Vandetanib Enhances Sensitivity to Tamoxifen in Estrogen Receptor Positive Breast Cancer
Abstract
Resistance to endocrine therapy (ET) is common in estrogen receptor (ER) positive breast cancer. Multiple studies have demonstrated that upregulation of MAPK signaling pathways contributes to ET resistance. Herein we show that vandetanib treatment enhances sensitivity to ET in ET-sensitive and -resistant ER+ breast cancer models. Vandetanib treatment alters the gene expression program of ER+ breast cancer cells resulting in a less proliferative and more estrogen responsive Luminal-A like character. Tyrosine kinase network reprogramming was assessed using multiplexed kinase inhibitor beads-mass spectrometry (MIB/MS) assay to identify adaptive resistance mechanisms to vandetanib treatment, including upregulation of HER2 activity. Co-treatment to inhibit HER2 with lapatinib enhanced sensitivity to vandetanib, demonstrating biologic activity of HER2 upregulation. Using a CRISPR knockout model, we demonstrate that vandetanib effects are partially mediated by RET receptor tyrosine kinase. Finally, we use our operating room-to-laboratory assay that measures drug response in individual primary tumor cells in short term cultures to demonstrate conserved gene expression changes, including increased HER2 activity signatures, in vandetanib treated cells, and identify features associated with vandetanib response. These results support future investigation of RET targeting strategies considering reprogrammed networks, such as activated HER2, in patients with ET resistant ER+ breast cancer.
Keywords: Breast cancer; endocrine resistance; functional proteomics; targeted therapeutics; tyrosine kinase inhibition.
Conflict of interest statement
Disclosure of conflict of interests: C.M.P is an equity stockholder and consultant of BioClassifier LLC; C.M.P is also listed as an inventor on patent applications for the Breast PAM50 Subtyping assay. The remaining authors declare no potential conflicts of interest.
Figures
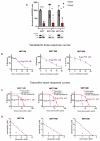



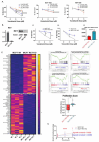

References
-
- Nabholtz JM, Tonkin K, Smylie M, Au HJ, Lindsay MA, and Mackey J. Chemotherapy of breast cancer: are the taxanes going to change the natural history of breast cancer? Expert Opin Pharmacother. 2000;1(2):187–206. - PubMed
-
- Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21(11):2101–9. - PubMed
-
- Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero JM, Freyer G, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30(22):2718–24. - PubMed
-
- Giaquinto AN, Sung H, Newman LA, Freedman RA, Smith RA, Star J, et al. Breast cancer statistics 2024. CA Cancer J Clin. 2024. - PubMed
-
- Harvey JM, Clark GM, Osborne CK, and Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–81. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
