Prioritization of Lipid Metabolism Targets for the Diagnosis and Treatment of Cardiovascular Diseases
- PMID: 39975574
- PMCID: PMC11836198
- DOI: 10.34133/research.0618
Prioritization of Lipid Metabolism Targets for the Diagnosis and Treatment of Cardiovascular Diseases
Abstract
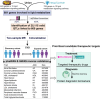
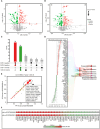
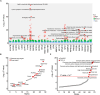
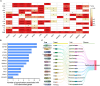
Background: Cardiovascular diseases (CVD) are a major global health issue strongly associated with altered lipid metabolism. However, lipid metabolism-related pharmacological targets remain limited, leaving the therapeutic challenge of residual lipid-associated cardiovascular risk. The purpose of this study is to identify potentially novel lipid metabolism-related genes by systematic genomic and phenomics analysis, with an aim to discovering potentially new therapeutic targets and diagnosis biomarkers for CVD. Methods: In this study, we conducted a comprehensive and multidimensional evaluation of 881 lipid metabolism-related genes. Using genome-wide association study (GWAS)-based mendelian randomization (MR) causal inference methods, we screened for genes causally linked to the occurrence and development of CVD. Further validation was performed through colocalization analysis in 2 independent cohorts. Then, we employed reverse screening using phenonome-wide association studies (PheWAS) and a drug target-drug association analysis. Finally, we integrated serum proteomic data to develop a machine learning model comprising 5 proteins for disease prediction. Results: Our initial screening yielded 54 genes causally linked to CVD. Colocalization analysis in validation cohorts prioritized this to 29 genes marked correlated with CVD. Comparison and interaction analysis identified 13 therapeutic targets with potential for treating CVD and its complications. A machine learning model incorporating 5 proteins for CVD prediction achieved a high accuracy of 96.1%, suggesting its potential as a diagnostic tool in clinical practice. Conclusion: This study comprehensively reveals the complex relationship between lipid metabolism regulatory targets and CVD. Our findings provide new insights into the pathogenesis of CVD and identify potential therapeutic targets and drugs for its treatment. Additionally, the machine learning model developed in this study offers a promising tool for the diagnosis and prediction of CVD, paving the way for future research and clinical applications.
Copyright © 2025 Zhihua Wang et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures
Similar articles
-
Investigating potential biomarkers of acute pancreatitis in patients with a BMI>30 using Mendelian randomization and transcriptomic analysis.Lipids Health Dis. 2024 Apr 22;23(1):119. doi: 10.1186/s12944-024-02102-3. Lipids Health Dis. 2024. PMID: 38649912 Free PMC article.
-
PTGES2 and RNASET2 identified as novel potential biomarkers and therapeutic targets for basal cell carcinoma: insights from proteome-wide mendelian randomization, colocalization, and MR-PheWAS analyses.Front Pharmacol. 2024 Jul 5;15:1418560. doi: 10.3389/fphar.2024.1418560. eCollection 2024. Front Pharmacol. 2024. PMID: 39035989 Free PMC article.
-
Deciphering the role of lipid metabolism-related genes in Alzheimer's disease: a machine learning approach integrating Traditional Chinese Medicine.Front Endocrinol (Lausanne). 2024 Oct 23;15:1448119. doi: 10.3389/fendo.2024.1448119. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39507054 Free PMC article.
-
Identifying new biomarkers and potential therapeutic targets for breast cancer through the integration of human plasma proteomics: a Mendelian randomization study and colocalization analysis.Front Endocrinol (Lausanne). 2024 Sep 16;15:1449668. doi: 10.3389/fendo.2024.1449668. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39351539 Free PMC article.
-
Identifying novel risk targets in inflammatory skin diseases by comprehensive proteome-wide Mendelian randomization study.Postgrad Med J. 2025 Mar 5:qgaf032. doi: 10.1093/postmj/qgaf032. Online ahead of print. Postgrad Med J. 2025. PMID: 40042804
Cited by
-
Revisiting the role of GDF15 in atherosclerosis in mouse and human.Acta Pharmacol Sin. 2025 Apr 30. doi: 10.1038/s41401-025-01561-3. Online ahead of print. Acta Pharmacol Sin. 2025. PMID: 40307459
References
LinkOut - more resources
Full Text Sources

