Stereochemical expression of Bi 6s2 lone pairs mediates fluoride-ion (De)insertion in tunnel-structured Bi2PdO4 and Bi1.6Pb0.4PtO4
- PMID: 39975768
- PMCID: PMC11834969
- DOI: 10.1039/d4sc08111k
Stereochemical expression of Bi 6s2 lone pairs mediates fluoride-ion (De)insertion in tunnel-structured Bi2PdO4 and Bi1.6Pb0.4PtO4
Abstract
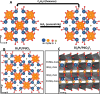
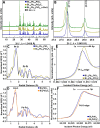
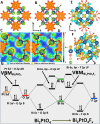
Fluoride-ion batteries are a promising alternative to lithium-ion batteries by dint of the greater crustal abundance of fluorine and the potential to alleviate the need for metal electrodeposition. However, conventional metal fluoride cathodes typically rely on conversion-type reactions that require propagation of a reaction-diffusion front, thereby limiting cycling performance and rate capability. In contrast, the topochemical insertion of fluoride-ions in periodic solids remains a relatively unexplored approach. Here, we explore the mechanisms of fluoridation of Bi2PdO4 and Bi1.6Pb0.4PtO4 insertion hosts that possess capacious tunnels that can accommodate fluoride-ions with a particular emphasis on elucidating the role of stereochemical expression of bismuth 6s2 lone pairs in mediating anion diffusion. We reveal that the topochemical solution-phase insertion and deinsertion of fluoride-ions at room temperature is mediated by redox reactions at platinum and palladium centers but involves multi-center synergies between d- and p-block atoms across the one-dimensional (1D) tunnel structure. While Pt and Pd centers mediate redox reactions, the stereochemically active lone pair electrons of Bi3+ play a pivotal role in facilitating reversible fluoride-ion diffusion. Consequently, Bi1.6Pb0.4PtO4 and Bi2PdO4 can be reversibly fluoridated with full recovery of the crystal lattice and with minimal alteration of the unit cell volume. The results reveal a key principle that the stereochemical activity of p-block electron lone pairs can be harnessed to modulate anion-lattice interactions and mediate facile anion diffusion.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Xu P. Tan D. H. S. Chen Z. Emerging Trends in Sustainable Battery Chemistries. Trends Chem. 2021;3(8):620–630. doi: 10.1016/j.trechm.2021.04.007. https://dx.doi.org/10.1016/j.trechm.2021.04.007 - DOI - DOI
-
- Zeng A. Chen W. Rasmussen K. D. Zhu X. Lundhaug M. Müller D. B. Tan J. Keiding J. K. Liu L. Dai T. Wang A. Liu G. Battery Technology and Recycling Alone Will Not Save the Electric Mobility Transition from Future Cobalt Shortages. Nat. Commun. 2022;13:1341. doi: 10.1038/s41467-022-29022-z. https://dx.doi.org/10.1038/s41467-022-29022-z - DOI - DOI - PMC - PubMed
-
- Andrews J. L. Banerjee S. It's Not Over until the Big Ion Dances: Potassium Gets Its Groove On. Joule. 2018;2(11):2194–2197. doi: 10.1016/j.joule.2018.11.003. https://dx.doi.org/10.1016/j.joule.2018.11.003 - DOI - DOI
-
- Choi J. W. Aurbach D. Promise and Reality of Post-Lithium-Ion Batteries with High Energy Densities. Nat. Rev. Mater. 2016;1(4):16013. doi: 10.1038/natrevmats.2016.13. https://dx.doi.org/10.1038/natrevmats.2016.13 - DOI - DOI
-
- Xiao A. W. Galatolo G. Pasta M. The Case for Fluoride-Ion Batteries. Joule. 2021;5(11):2823–2844. doi: 10.1016/j.joule.2021.09.016. https://dx.doi.org/10.1016/j.joule.2021.09.016 - DOI - DOI
LinkOut - more resources
Full Text Sources

