This is a preprint.
Feedback control over plasma drug concentrations achieves rapid and accurate control over solid-tissue drug concentrations
- PMID: 39975897
- PMCID: PMC11838736
- DOI: 10.21203/rs.3.rs-5868915/v1
Feedback control over plasma drug concentrations achieves rapid and accurate control over solid-tissue drug concentrations
Update in
-
Feedback Control over Plasma Drug Concentrations Achieves Rapid and Accurate Control over Solid-Tissue Drug Concentrations.ACS Pharmacol Transl Sci. 2025 Apr 11;8(5):1416-1423. doi: 10.1021/acsptsci.5c00142. eCollection 2025 May 9. ACS Pharmacol Transl Sci. 2025. PMID: 40370982
Abstract
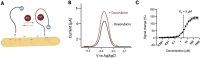
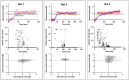
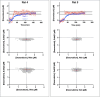
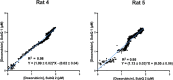
Electrochemical aptamer-based (EAB) sensors enable the continuous, real-time monitoring of drugs and biomarkers in situ in the blood, brain, and peripheral tissues of live subjects. The real-time concentration information produced by these sensors provides unique opportunities to perform closed-loop, feedback-controlled drug delivery, by which the plasma concentration of a drug can be held constant or made to follow a specific, time-varying profile. Motivated by the observation that the site of action of many drugs is the solid tissues and not the blood, here we experimentally confirm that maintaining constant plasma drug concentrations also produces constant concentrations in the interstitial fluid (ISF). Using an intravenous EAB sensor we performed feedback control over the concentration of doxorubicin, an anthracycline chemotherapeutic, in the plasma of live rats. Using a second sensor placed in the subcutaneous space, we find drug concentrations in the ISF rapidly (30-60 min) match and then accurately (RMS deviation of 8-21%) remain at the feedback-controlled plasma concentration, validating the use of feedback-controlled plasma drug concentrations to control drug concentrations in the solid tissues that are the site of drug action. We expanded to pairs of sensors in the ISF, the outputs of the individual sensors track one another with good precision (R 2 = 0.95-0.99), confirming that the performance of in vivo EAB sensors matches that of prior, in vitro validation studies. These observations suggest EAB sensors could prove a powerful new approach to the high-precision personalization of drug dosing.
Conflict of interest statement
Competing interests: K.W.P. owns equity in and consults for a company that is commercializing in vivo EAB sensors. Following the completion of this work, J.G. and K.K.L. became employees at a company that is commercializing in vivo EAB sensors. All other authors declare that they have no competing interests.
Figures

References
-
- Arroyo-Currás N. et al. Subsecond-Resolved Molecular Measurements in the Living Body Using Chronoamperometrically Interrogated Aptamer-Based Sensors. ACS Sens 3, 360–366 (2018). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

