This is a preprint.
Human Organ Chips Reveal New Inflammatory Bowel Disease Drivers
- PMID: 39975910
- PMCID: PMC11838723
- DOI: 10.21203/rs.3.rs-5627712/v1
Human Organ Chips Reveal New Inflammatory Bowel Disease Drivers
Abstract
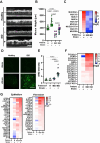
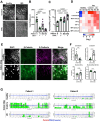
Inflammatory bowel disease (IBD) patients exhibit compromised intestinal barrier function and decreased mucus accumulation, as well as increased inflammation, fibrosis, and cancer risk, with symptoms often being exacerbated in women during pregnancy. Here, we show that these IBD hallmarks can be replicated using human Organ Chips lined by IBD patient-derived colon epithelial cells interfaced with matched fibroblasts cultured under flow. Use of heterotypic tissue recombinants revealed that IBD fibroblasts are the primary drivers of multiple IBD symptoms. Inflammation and fibrosis are accentuated by peristalsis-like motions in IBD Chips and when exposed to pregnancy-associated hormones in female IBD Chips. Carcinogen exposure also increases inflammation, gene mutations, and chromosome duplication in IBD Chips, but not in Healthy Chips. These data enabled by human Organ Chip technology suggest that the intestinal stroma, sex hormones, and peristalsis-associated mechanical deformations play a key role in driving inflammation, fibrosis, and disease progression in male and female IBD patients.
Keywords: Carcinogenesis; Crohn’s Disease; Epithelial-Stromal Interactions; Organ-on-a-chip; Ulcerative Colitis; Women’s Health.
Conflict of interest statement
Competing interests D.E.I. holds equity in Emulate, chairs its scientific advisory board and is a member of its board of directors. The other authors declare no competing interests.
Figures



References
-
- Watermeyer G. Pregnancy and inflammatory bowel disease. South African Gastroenterology Review 5, 4–6 (2007).
-
- Ingber D. E. Cancer as a disease of epithelial–mesenchymal interactions and extracellular matrix regulation. Differentiation 70, 547–560 (2002). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

