Sodium benzoate for the treatment of hepatic encephalopathy in humans and animals: a systematic review and meta-analysis
- PMID: 39975997
- PMCID: PMC11867799
- DOI: 10.1097/MEG.0000000000002911
Sodium benzoate for the treatment of hepatic encephalopathy in humans and animals: a systematic review and meta-analysis
Abstract
Background and aim: Hepatic encephalopathy (HE) is a life-threatening condition where brain function is impaired mainly due to high systemic ammonia levels. HE is associated with a high 1-year mortality. No universally accepted guidelines for the treatment of HE exist. Nitrogen scavengers, such as sodium benzoate (SB), have been proven very effective to treat hyperammonemia in patients with urea cycle defects, in acute and chronic settings. We hypothesized that SB can also be an effective treatment of HE caused by end-stage liver disease or portosystemic shunting, as long as liver function is partially intact. The aim of this meta-analysis is to study the effect of SB in humans and animals with HE due to end-stage liver disease or portosystemic shunting.
Methods: Embase, Medline (Ovid and PubMed), Web-of-Science, Cochrane, and Google Scholar were searched on 19 July 2021, both human and animal studies were eligible.
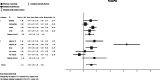
Results: Sixteen studies were included, consisting of four clinical trials, five animal studies, and seven case reports, including 314 subjects. Meta-analysis included 284 subjects. The standardized mean difference (SMD) of SB's ammonia-lowering effect was 0.89 SMD [95% confidence interval (CI): 0.27-1.51] in clinical trials and 1.63 SMD (95% CI: -0.12 to 3.39) in animal studies. Considerable heterogeneity was present in the included studies.
Conclusion: SB seems to be an effective treatment for HE or hyperammonemia caused by end-stage liver disease or portosystemic shunting. However, additional high-quality studies are necessary for more robust conclusions.
Keywords: ammonia scavengers; cirrhosis; end-stage liver disease; hepatic encephalopathy; hyperammonemia; portosystemic shunt; sodium benzoate.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Cagnon L, Braissant O. Hyperammonemia-induced toxicity for the developing central nervous system. Brain Res Rev. 2007; 56:183–197. - PubMed
-
- Norenberg MD, Rao KV, Jayakumar AR. Mechanisms of ammonia-induced astrocyte swelling. Metab Brain Dis. 2005; 20:303–318. - PubMed
-
- Wijdicks EFM. Hepatic encephalopathy. N Engl J Med. 2016; 375:1660–1670. - PubMed
-
- Tapper EB, Jiang ZG, Patwardhan VR. Refining the ammonia hypothesis: a physiology-driven approach to the treatment of hepatic encephalopathy. Mayo Clin Proc. 2015; 90:646–658. - PubMed
-
- Fichet J, Mercier E, Genée O, Garot D, Legras A, Dequin PF, Perrotin D. Prognosis and 1-year mortality of intensive care unit patients with severe hepatic encephalopathy. J Crit Care. 2009; 24:364–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources