Role of single-nucleotide polymorphism microarray in the classification of BAP1-inactivated melanocytic tumours
- PMID: 39976080
- PMCID: PMC12129603
- DOI: 10.1111/his.15434
Role of single-nucleotide polymorphism microarray in the classification of BAP1-inactivated melanocytic tumours
Abstract
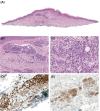
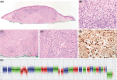
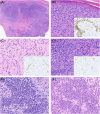
Aims: BAP1-inactivated melanocytic tumours (BIMTs) occur sporadically and in association with a familial tumour predisposition syndrome involving germline mutations in the BRCA1-associated protein-1 (BAP1) gene. Here we report the clinical features, histopathologic findings, and chromosomal copy number data of 19 BAP1-inactivated melanocytomas (BIMs) and compare their features to those of five BAP1-inactivated melanomas.
Methods: We retrospectively searched the Department of Pathology archives and EMERSE (Electronic Medical Record Search Engine) for BIMTs that had undergone single-nucleotide polymorphism (SNP) microarray testing. Clinical history/follow-up data, detailed histopathologic features, and SNP microarray results were recorded.
Results: Overall, four patients (4/13) with BIMs and available clinical history had features suspicious for a germline BAP1 aberration. In BIMs (19 cases), the BAP1-inactivated component showed variable cytologic features, including epithelioid (predominant), rhabdoid, plasmacytoid, and nevoid morphologies. Sentinel lymph node biopsy was negative in all (6/6) patients in which this procedure was performed. No patient diagnosed with a BIM with available clinical follow-up (18/19; mean 38 months) experienced an adverse event. While the histologic appearances of the five melanomas varied, one case resembled a BIM. The median mitotic count was 1/mm2 (range 0-6 mm2) in BIMs compared to 3/mm2 (range 1-4/mm2) in melanomas (P = 0.04). The median number of copy number alterations (CNAs) was two (range 0-6) in indolent cases versus seven (range 6-10) in melanomas (P = 0.0005). The most common molecular aberration after loss of 3p21 was heterozygous loss of the CDKN2A locus, which unlike homozygous loss has not been associated with melanoma.
Conclusion: While most BIMs appear to have a favourable prognosis, even those with multiple CNAs, they deserve careful integration of all clinical and pathologic findings. Although not fully diagnostic, a mitotic count of ≥3/mm2 and ≥6 CNAs in the appropriate context is supportive of a diagnosis of BAP1-inactivated melanoma.
Keywords: BAP1‐inactivated melanocytoma; chromosomal microarray analysis; melanoma; single nucleotide polymorphism microarray.
© 2025 The Author(s). Histopathology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


References
-
- Shain AH, Yeh I, Kovalyshyn I et al. The genetic evolution of melanoma from precursor lesions. N Engl J Med 2015; 373; 1926–1936. - PubMed
-
- Alomari AK, Miedema JR, Carter MD et al. DNA copy number changes correlate with clinical behavior in melanocytic neoplasms: proposal of an algorithmic approach. Mod Pathol 2020; 33; 1307–1317. - PubMed
-
- WHO Classification of Tumours Editorial Board . Skin tumours [Internet; beta version ahead of print]. Lyon (France): International Agency for Research on Cancer 2023; (WHO classification of tumours series, 5th ed.; vol. 12). Available from: https://tumourclassification.iarc.who.int/chapters/64.
-
- Andea AA. Molecular testing for melanocytic tumors: a practical update. Histopathology 2022; 80; 150–165. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

