Blockade of the PGE2 Pathway Inhibits the Growth of PTEN-Deficient HNSCC Tumors
- PMID: 39976130
- PMCID: PMC12137011
- DOI: 10.1158/1535-7163.MCT-24-0604
Blockade of the PGE2 Pathway Inhibits the Growth of PTEN-Deficient HNSCC Tumors
Abstract
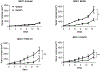
Increased PI3K signaling as a result of PIK3CA mutation or amplification or decreased expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is one of the most common alterations in head and neck squamous cell carcinoma (HNSCC). PTEN negatively regulates PI3K signaling and its downstream effectors including COX2. COX2 mediates the synthesis of prostaglandin E2 (PGE2) which contributes to immunosuppression in the tumor microenvironment. PGE2 also binds to one or more EP receptors (EP1-EP4) and promotes the growth of tumor cells via activation of EP2 and EP4. However, the role of PGE2 in PTEN-deficient HNSCC is incompletely understood. In this study, we assessed PGE2 signaling in PTEN-deficient HNSCC and evaluated the effect of aspirin or TPST-1495, a dual EP2/EP4 antagonist, on the growth of PTEN knockout and PIK3CA-altered HNSCC tumors in immunocompetent mice. Our results demonstrated that aspirin selectively inhibits the growth of PTEN knockout HNSCC tumors. TPST-1495 inhibited tumor growth and substantially increased the antitumor activity of the immune checkpoint inhibitor anti-PD1. To date, there are no FDA-approved therapies for PI3K pathway-altered HNSCC. Our findings suggest that NSAIDs demonstrate antitumor activity in PTEN-deficient or PI3K-altered tumors whereas EP2/EP4 targeting may augment FDA-approved anti-PD1 therapy in HNSCC.
©2025 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest Disclosure: Tempest Therapeutics provided partial funding for this study (J.R. Grandis). All other authors have no conflicts of interest to declare.
Figures

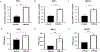
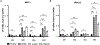
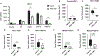
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

