Traits determine dispersal and colonization abilities of microbes
- PMID: 39976438
- PMCID: PMC11921345
- DOI: 10.1128/aem.02055-24
Traits determine dispersal and colonization abilities of microbes
Abstract
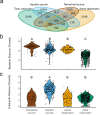
Many microbes disperse through the air, yet the phenotypic traits that enhance or constrain aerial dispersal or allow successful colonization of new habitats are poorly understood. We used a metabarcoding bacterial and eukaryotic data set to explore the trait structures of the aquatic, terrestrial, and airborne microbial communities near the Salton Sea, California, as well as those colonizing a series of experimental aquatic mesocosms. We assigned taxonomic identities to amplicon sequence variants (ASVs) and matched them to functional trait values through published papers and databases that infer phenotypic and/or metabolic traits information from taxonomy. We asked what traits distinguish successful microbial dispersers and/or colonizers from terrestrial and aquatic source communities. Our study found broad differences in taxonomic and trait composition between dispersers and colonizers compared to the source soil and water communities. Dispersers were characterized by larger cell diameters, colony formation, and fermentation abilities, while colonizers tended to be phototrophs that form mucilage and have siliceous coverings. Shorter population doubling times, spore-, and/or cyst-forming organisms were more abundant among the dispersers and colonizers than the sources. These results show that the capacity for aerial dispersal and colonization varies among microbial functional groups and taxa and is related to traits that affect other functions like resource acquisition, predator avoidance, and reproduction. The ability to disperse and colonize new habitats may therefore distinguish microbial guilds based on tradeoffs among alternate ecological strategies.IMPORTANCEMicrobes have long been thought to disperse rapidly across biogeographic barriers; however, whether dispersal or colonization vary among taxa or groups or is related to cellular traits remains unknown. We use a novel approach to understand how microorganisms disperse and establish themselves in different environments by looking at their traits (physiology, morphology, life history, and behavior characteristics). By collecting samples from habitats including water, soil, and the air and colonizing experimental tanks, we found dispersal and invasion vary among microorganisms. Some taxa and functional groups are found more often in the air or colonizing aquatic environments, while others that are commonly found in the soil or water rarely disperse or invade new habitat. Interestingly, the traits that help microorganisms survive and thrive also play a role in their ability to disperse and colonize. These findings have significant implications for understanding microorganisms' success and adaptation to new environments.
Keywords: aerosols; airborne; community assembly; trait tradeoff.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Baas Becking LGM. 1934. Geobiologie of inleiding tot de milieukunde. The Hague, the Netherlands.
-
- Rodó X, Pozdniakova S, Borràs S, Matsuki A, Tanimoto H, Armengol M-P, Pey I, Vila J, Muñoz L, Santamaria S, Cañas L, Morguí J-A, Fontal A, Curcoll R. 2024. Microbial richness and air chemistry in aerosols above the PBL confirm 2,000-km long-distance transport of potential human pathogens. Proc Natl Acad Sci USA 121:e2404191121. doi:10.1073/pnas.2404191121 - DOI - PMC - PubMed
-
- Villarino E, Watson JR, Chust G, Woodill AJ, Klempay B, Jonsson B, Gasol JM, Logares R, Massana R, Giner CR, Salazar G, Alvarez‐Salgado XA, Catala TS, Duarte CM, Agusti S, Mauro F, Irigoien X, Barton AD. 2022. Global beta diversity patterns of microbial communities in the surface and deep ocean. Global Ecol Biogeogr 31:2323–2336. doi:10.1111/geb.13572 - DOI

