Mind the Breath: Feasibility of Capnography-Assisted Learned Monitored (CALM) Breathing for Dyspnea Treatment
- PMID: 39976559
- PMCID: PMC11864056
- DOI: 10.1097/HCR.0000000000000939
Mind the Breath: Feasibility of Capnography-Assisted Learned Monitored (CALM) Breathing for Dyspnea Treatment
Abstract
Purpose: To evaluate the feasibility and acceptability of Capnography-Assisted Learned Monitored (CALM) Breathing, a carbon dioxide (CO 2 ) biofeedback, and motivational interviewing intervention, to treat dyspnea and anxiety together.
Methods: We randomized adults (n = 42) with chronic obstructive pulmonary disease (COPD) to a 4-week, 8-session intervention (CALM Breathing, n = 20) or usual care (n = 22). The CALM Breathing intervention consisted of tailored, slow nasal breathing exercises, capnography biofeedback, motivational interviewing, and a home breathing exercise program. The intervention targeted unlearning dysfunctional breathing behaviors. All participants were offered outpatient pulmonary rehabilitation (PR) in the second phase of the study. The primary outcomes were feasibility and acceptability of CALM Breathing. Exploratory secondary outcomes included respiratory and mood symptoms, physiological and exercise tolerance measures, quality of life, and PR uptake.
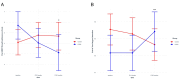
Results: Attendance at CALM Breathing sessions was 84%, dropout was 5%, and home exercise completion was 90% and 73% based on paper and device logs, respectively. Satisfaction with CALM Breathing therapy was rated as "good" to "excellent" by 92% of participants. Significantly greater between-group improvements in secondary outcomes-respiratory symptoms, activity avoidance, oxygen saturation (SpO 2 ), end-tidal CO 2 , and breathing self-regulation (interoception)-were found post-intervention at 6 weeks in support of CALM Breathing compared with usual care. At 3 months (after PR initiation), statistically significant between-group differences in Borg dyspnea and SpO 2 post-6-minute walk test were identified also supporting CALM Breathing.
Conclusions: Patient-centered CALM Breathing was feasible and acceptable in adults with COPD and dyspnea anxiety. A CALM Breathing intervention may optimize dyspnea treatment and complement PR.
Trial registration: ClinicalTrials.gov NCT04786184.
Copyright © 2025 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

