Efficacy and safety of patiromer for hyperkalemia: a randomized, placebo-controlled phase 3 study
- PMID: 39976633
- PMCID: PMC12204919
- DOI: 10.1007/s10157-025-02637-4
Efficacy and safety of patiromer for hyperkalemia: a randomized, placebo-controlled phase 3 study
Abstract
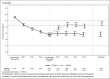
Background: This is the phase 3 study in Japan designed to verify the superiority of patiromer over placebo using the change in serum potassium level (sK-level).
Methods: This study was a multicenter, randomized withdrawal study targeting Japanese hyperkalemic patients. It consisted of the run-in period and the double-blind period. The run-in period was an active single-arm, open-label period (4 or 5 weeks). The double-blind period was a randomized, placebo-controlled, parallel-group, double-blind period (4 weeks). Patients whose sK-level was within the normal range at week 4 or 5 of the run-in period entered the double-blind period. Patients who entered the double-blind period were randomly assigned to the patiromer group or the placebo group.
Results: As a result of the primary analysis, the change of the sK-level (95% CI) from baseline to week 4 in the double-blind-period, was - 0.02 (- 0.19, 0.15) mmol/L in the patiromer group, and 0.78 (0.60, 0.96) mmol/L in the placebo group, with a statistically significant difference between the two treatment groups (p < 0.001). Similarly, statistically significant differences were also observed between the two groups at weeks 1, 2, and 3. Furthermore, the proportion of patients whose sK-level was maintained within the normal range were statistically significantly higher in the patiromer group than in the placebo group at all time points. No adverse events requiring particular attention were observed.
Conclusion: Patiromer can improve hyperkalemia by lowering sK-level and can suppress the recurrence of hyperkalemia with continued administration, and is safe and easy-to-use for a wide range of patients.
Keywords: Hyperkalemia; Patiromer; Phase III; Placebo-controlled.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: N Kashihara has received consulting fees from AstraZeneca, Gilead Sciences, Kyowa Kirin, and GlaxoSmithKline, has received honoraria for lectures from Daiichi Sankyo, Takeda Pharmaceutical, Astellas Pharma, Otsuka Pharmaceutical, and Kyowa Kirin, and has received research grants from Mitsubishi Tanabe Pharma, Toray Industries, Baxter, Chugai Pharmaceutical, Kyowa Kirin, Bayer Pharm, Ono Pharm, Daiichi Sankyo, Nippon Boehringer Ingelheim, Astellas Pharma, and Takeda Pharmaceutical. H Okada has received honoraria for lectures from Kyowa Kirin, Daiichi Sankyo, AstraZeneca, Torii Pharm., Mitsubishi Tanabe Pharm, Boehringer Ingelheim, Ono Pharm, Eli Lilly Pharm, has received research grants from Kyowa Kirin, Bayer Pharm, Chugai Pharm, and has received subsidies or donations from Chugai Pharm., Ono Pharm., Kyowa Kirin, and Bayer Pharm. Y Suzuki, T Iwamoto, M Yasutomi, and M Matsui have no conflicts of interest to declare. R Takezawa, T Ishii and Y Tomioka are employees of Zeria Pharmaceuticals.
Figures
References
-
- Japanese Society of Nephrology: Evidence-based clinical practice guideline for CKD 2023. Tokyo Igakusha, Tokyo, 2023. (in Japanese)
-
- Tsutsui H, Isobe M, Ito H, et al. JCS 2017/JHFS 2017 Guidelines on diagnosis and treatment of acute and chronic heart failure. Circ J. 2019;83(10):2084–184. - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Working Group. KDIGO 2021 Clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3S):S1–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials