Health-Related Quality of Life and Cognitive Performance During 12-Month Adjunctive Brivaracetam Treatment in Patients with Focal-Onset Seizures: A Prospective, Observational Study in Europe
- PMID: 39976892
- PMCID: PMC11906959
- DOI: 10.1007/s40120-024-00698-3
Health-Related Quality of Life and Cognitive Performance During 12-Month Adjunctive Brivaracetam Treatment in Patients with Focal-Onset Seizures: A Prospective, Observational Study in Europe
Abstract
Introduction: This analysis aimed to evaluate patient-related outcomes for health-related quality of life (HRQoL) and cognitive performance in patients (≥ 16 years) with focal-onset seizures (FOS), with/without focal to bilateral tonic-clonic seizures, after initiating adjunctive brivaracetam (BRV) in routine clinical practice.
Methods: A 12-month, prospective, real-world, noninterventional study in nine European countries (EP0077/NCT02687711) was performed. BRV was prescribed per clinical practice and the European Summary of Product Characteristics. The outcomes evaluated were the Patient Weighted Quality of Life in Epilepsy Inventory-Form 31 (QOLIE-31-P), the Clinical and the Patient's Global Impression of Change (CGIC and PGIC, respectively), and EpiTrack®. EpiTrack® scores were categorized into cognitive performance categories (excellent: ≥ 39 points; average: 32-38 points; mildly impaired: 29-31 points; significantly impaired: ≤ 28 points). The change in EpiTrack® score was evaluated [improvement: increase in score of ≥ 4 points; no change: change in score of - 2 to 3 points (inclusive); worsening: change in score of at least - 3 points].
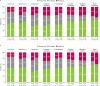
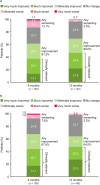
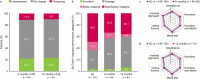
Results: Full Analysis Set: 541 patients. 46.6% of patients reported a clinically meaningful improvement in QOLIE-31-P total score from baseline to 12 months; the mean change in total score was + 6.2 points (N = 103). Per CGIC (N = 142) and PGIC (N = 148), respectively, 69.0% and 62.8% of patients had improved in overall condition at 12 months versus baseline, while 3.5% and 8.1% had worsened. EpiTrack® categories at 12 months versus baseline showed improved cognitive performance [baseline (N = 142): significantly impaired 49.3%, mildly impaired 14.8%, average 33.1%, excellent 2.8%; 12 months (N = 61): significantly impaired 36.1%, mildly impaired 4.9%, average 52.5%, excellent 6.6%]. At 12 months, 67.2% of patients showed no significant change from baseline in EpiTrack® score, 23.0% had improved, and 9.8% had worsened (N = 61).
Conclusion: In patients with predominantly difficult-to-treat FOS, BRV add-on was associated with good HRQoL and cognitive functioning. Cognitive functioning remained stable for 12 months after BRV initiation in most patients; nearly one-quarter experienced significant improvements. At 12 months, 46.6% of patients reported clinically meaningful HRQoL improvements, and most showed an improved overall condition.
Keywords: Brivaracetam; Cognitive performance; EpiTrack; Epilepsy; Focal-onset seizure; Health-related quality of life; Noninterventional; Observational; Real-world.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Eduardo Rubio-Nazabal has received honoraria from BIAL, Eisai, and UCB for speaking. Marian Majoie has received financial compensation through her institution for participating in contract research from Eisai, GW Pharmaceuticals, UCB, and Zogenix. Anne-Liv Schulz, Fiona Brock, Iryna Leunikava, and Dimitrios Bourikas are employees of UCB. Anne-Liv Schulz and Dimitrios Bourikas have received UCB stocks from their employment. Bernhard J. Steinhoff has received honoraria for consulting (from Angelini Pharma, Arvelle Therapeutics, B. Braun Melsungen, Desitin, Eisai, GW Pharmaceuticals, Neuraxpharm, and UCB), for serving on a scientific advisory board (from Arvelle Therapeutics, GW Pharmaceuticals, UCB, and Zogenix), and for speaking (from Arvelle Therapeutics, Desitin, Eisai, Hikma Pharmaceuticals, UCB, and Zogenix), and has received research support from Eisai, SK Life Science, and UCB. Ethical Approval: The study protocol was reviewed and approved by an Institutional Review Board/Independent Ethics Committee (see Institutional Review Board/Independent Ethics Committee appendix in the Supplementary Material), per country-specific regulations. Written data consent was obtained from the patient, parent(s), or legal representative before study participation, in accordance with local regulations and the Declaration of Helsinki.
Figures

References
-
- Saadi A, Patenaude B, Mateen FJ. Quality of life in epilepsy—31 inventory (QOLIE-31) scores: a global comparison. Epilepsy Behav. 2016;65:13–7. - PubMed
-
- Jalava M, Sillanpää M, Camfield C, Camfield P. Social adjustment and competence 35 years after onset of childhood epilepsy: a prospective controlled study. Epilepsia. 1997;38(6):708–15. - PubMed
-
- Wiebe S, Bellhouse DR, Fallahay C, Eliasziw M. Burden of epilepsy: the Ontario Health Survey. Can J Neurol Sci. 1999;26(4):263–70. - PubMed
-
- Wagner AK, Bungay KM, Kosinski M, Bromfield EB, Ehrenberg BL. The health status of adults with epilepsy compared with that of people without chronic conditions. Pharmacotherapy. 1996;16(1):1–9. - PubMed
-
- Taylor RS, Sander JW, Taylor RJ, Baker GA. Predictors of health-related quality of life and costs in adults with epilepsy: a systematic review. Epilepsia. 2011;52(12):2168–80. - PubMed
LinkOut - more resources
Full Text Sources

