Selective Laser Trabeculoplasty After Medical Treatment for Glaucoma or Ocular Hypertension
- PMID: 39976961
- PMCID: PMC11843460
- DOI: 10.1001/jamaophthalmol.2024.6492
Selective Laser Trabeculoplasty After Medical Treatment for Glaucoma or Ocular Hypertension
Abstract
Importance: Primary selective laser trabeculoplasty (SLT) is a safe primary treatment for open-angle glaucoma (OAG) and ocular hypertension (OHT). However, there is limited evidence on its use as a secondary treatment, ie, after prior use of ocular hypotensive eye drops.
Objective: To evaluate outcomes following SLT after using hypotensive eye drops for at least 3 years.
Design, setting, and participants: This is a post hoc exploratory analysis of data from a multicenter randomized clinical trial conducted within the UK National Health Service. Participants were patients with OAG or OHT who participated in the LiGHT trial. Data were analyzed from February 2021 to December 2024.
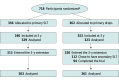
Intervention: Participants were initially randomized to either primary SLT or primary hypotensive eye drops and remained on the allocated treatment pathway for 3 years. Participants using eye drops were then allowed to have secondary SLT as a treatment switch (to reduce their medication load) or as a treatment escalation (if more intense treatment was needed). Participants were treated and monitored according to a predefined protocol.
Main outcomes and measures: The outcomes of interest were rates of incisional glaucoma surgery, medication use, and intraocular pressure.
Results: In total, 633 participants entered the extension of the LiGHT trial, and 524 participants (82.8%) completed the extension (72 months). Of 320 participants receiving primary hypotensive eye drops, 112 (35.0%) received SLT: 70 participants switched to SLT, 29 participants had SLT as a treatment escalation, and 13 participants had SLT as a treatment escalation in 1 eye and as a treatment switch in the other eye. Switching to SLT was associated with a reduction in the number of medications (mean [SD], 1.38 [0.62] to 0.59 [0.92] active ingredients; mean difference, 0.79 [95% CI 0.66 to 0.93] active ingredients; P < .001). At 72 months, 69 eyes that switched to SLT (60.5%) needed no medical or surgical treatment, and 62 eyes receiving 1 drug before switching (83.8%) needed no medical treatment. Escalating to SLT was associated with a mean intraocular pressure reduction of 4.6 mm Hg (21.8%), and 30 eyes (62.5%) reached target intraocular pressure at 72 months without the need for surgery; 9 eyes (18.7%) needed a trabeculectomy.
Conclusions and relevance: This secondary analysis of a randomized clinical trial found that secondary SLT was associated with a reduction in the medication load for stable, medically treated eyes. For medically uncontrolled eyes, there is evidence that SLT could provide additional intraocular pressure control, but the need for trabeculectomy was not eliminated.
Trial registration: isrctn.org Identifier: ISRCTN32038223.
Conflict of interest statement
Figures

Comment on
-
Shedding LiGHT on Selective Laser Trabeculoplasty.JAMA Ophthalmol. 2025 Apr 1;143(4):302-303. doi: 10.1001/jamaophthalmol.2025.0004. JAMA Ophthalmol. 2025. PMID: 39976932 No abstract available.
References
-
- Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. ; LiGHT Trial Study Group . Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393(10180):1505-1516. doi:10.1016/S0140-6736(18)32213-X - DOI - PMC - PubMed
-
- European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br J Ophthalmol. 2021;105(suppl 1):1-169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

