Adeno-Associated Virus Gene Therapy Development: Early Planning and Regulatory Considerations to Advance the Platform Vector Gene Therapy Program
- PMID: 39976996
- PMCID: PMC11971537
- DOI: 10.1089/hum.2024.230
Adeno-Associated Virus Gene Therapy Development: Early Planning and Regulatory Considerations to Advance the Platform Vector Gene Therapy Program
Abstract
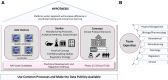
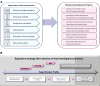
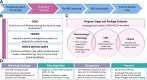
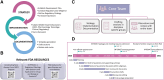
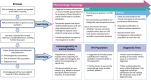
Gene therapy development presents multiple challenges, and early planning is vital in the successful implementation of such programs. The Platform Vector Gene Therapy (PaVe-GT) program is a National Institutes of Health (NIH) initiative developing adeno-associated virus (AAV) gene therapies for four low-prevalence rare diseases. Utilizing the platform-based approach, the program aims to incorporate efficiencies throughout the preclinical and clinical development processes followed by public dissemination of scientific and regulatory learnings. Early in development, the establishment of a Target Product Profile (TPP) by the research team is a critical step to guide product development and align preclinical studies to clinical objectives. Based on the specific needs of the investigational product as defined in the TPP, an overall regulatory strategy can then be outlined to meet the regulatory requirements for the first-in-human clinical trials. During the preclinical phase of development, sponsors may request meetings with the Food and Drug Administration (FDA) to gather feedback on the planned studies and regulatory strategy. To pave the way for PaVe-GT's first investigational AAV gene therapy lead candidate, AAV9-hPCCA, we sought early feedback from the FDA utilizing an INitial Targeted Engagement for Regulatory Advice on CBER/CDER ProducTs (INTERACT) meeting. Here, we elaborate on the value of establishing a TPP and the FDA INTERACT meeting by including our initial AAV9-hPCCA TPP, detailing our INTERACT meeting experience, providing all corresponding regulatory documentation, and highlighting lessons learned. The regulatory documents along with templates developed by our program can also be found on the PaVe-GT website (https://pave-gt.ncats.nih.gov/). This communication aims to provide stakeholders with resources that can be applied to drug development programs in establishing a viable regulatory path to clinical trial initiation.
Keywords: AAV9; INTERACT meeting; PCCA; PaVe-GT; gene therapy; propionic acidemia; rare diseases; target product profile.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
