Extended Continuous Positive Airway Pressure in Preterm Infants Increases Lung Growth at 6 Months: A Randomized Controlled Trial
- PMID: 39977011
- PMCID: PMC12005030
- DOI: 10.1164/rccm.202411-2169OC
Extended Continuous Positive Airway Pressure in Preterm Infants Increases Lung Growth at 6 Months: A Randomized Controlled Trial
Abstract
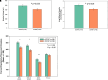
Rationale: Extended continuous positive airway pressure (eCPAP) in the neonatal ICU (NICU) for stable preterm infants increases lung volumes. Its effect on lung growth after discharge is unknown. Objectives: To assess whether 2 weeks of eCPAP in stable preterm infants is associated with increased alveolar volume (Va) at 6 months corrected age. Methods: This randomized controlled trial was conducted at Oregon Health & Science University. Outpatient assessors were unaware of treatment assignment. One hundred infants were randomized to eCPAP versus CPAP discontinuation (dCPAP) to room air. Measurements and Main Results: The primary outcome was Va by the single breath hold technique at 6 months corrected age. Secondary outcomes included DlCO and forced expiratory flows (FEFs). FRC was measured in the NICU. Infants randomized to eCPAP (n = 54) versus dCPAP (n = 46) had the following measurements shown as adjusted mean (SE): Va (500.2 [24.9] vs. 418.1 [23.4] ml; adjusted mean difference, 82.1 [95% confidence interval (CI), 8.3-155.9]; P = 0.033); DlCO (3.4 [0.2] vs. 2.8 [0.1] ml/min/mm Hg; adjusted mean difference, 0.6 [95% CI, 0.1-1.1]; P = 0.018); measurement of FEF at 50% of the expired volume (500.6 [18.2] vs. 437.9 [17.9] ml/s; adjusted mean difference, 62.7 [95% CI, 4.5-121.0]; P = 0.039); FEF between 25% and 75% of expired volume (452.0 [17.4] vs. 394.4 [17.4] ml/s; adjusted mean difference, 57.5 [95% CI, 1.3-113.8]; P = 0.046). Conclusions: Infants randomized to eCPAP versus dCPAP had significantly increased Va at 6 months corrected age. DlCO and FEFs were also increased. Extending CPAP in stable preterm infants in the NICU may be a nonpharmacologic and safe therapy to promote lung growth. Clinical trial registered with www.clinicaltrials.gov (NCT04295564).
Keywords: airway function; alveolar volume; lung diffusion capacity; nasal continuous positive airway pressure; preterm infants.
Figures


Comment in
-
Extending Nasal Continuous Positive Airway Pressure for Preterm Infants: New Targets for an Old Device.Am J Respir Crit Care Med. 2025 Apr;211(4):550-551. doi: 10.1164/rccm.202502-0342ED. Am J Respir Crit Care Med. 2025. PMID: 39998443 Free PMC article. No abstract available.
References
-
- Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med . 2013;1:728–742. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

