A novel quinone biosynthetic pathway illuminates the evolution of aerobic metabolism
- PMID: 39977315
- PMCID: PMC11874023
- DOI: 10.1073/pnas.2421994122
A novel quinone biosynthetic pathway illuminates the evolution of aerobic metabolism
Abstract
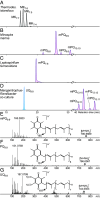
The dominant organisms in modern oxic ecosystems rely on respiratory quinones with high redox potential (HPQs) for electron transport in aerobic respiration and photosynthesis. The diversification of quinones, from low redox potential (LPQ) in anaerobes to HPQs in aerobes, is assumed to have followed Earth's surface oxygenation ~2.3 billion years ago. However, the evolutionary origins of HPQs remain unresolved. Here, we characterize the structure and biosynthetic pathway of an ancestral HPQ, methyl-plastoquinone (mPQ), that is unique to bacteria of the phylum Nitrospirota. mPQ is structurally related to the two previously known HPQs, plastoquinone from Cyanobacteriota/chloroplasts and ubiquinone from Pseudomonadota/mitochondria, respectively. We demonstrate a common origin of the three HPQ biosynthetic pathways that predates the emergence of Nitrospirota, Cyanobacteriota, and Pseudomonadota. An ancestral HPQ biosynthetic pathway evolved ≥ 3.4 billion years ago in an extinct lineage and was laterally transferred to these three phyla ~2.5 to 3.2 billion years ago. We show that Cyanobacteriota and Pseudomonadota were ancestrally aerobic and thus propose that aerobic metabolism using HPQs significantly predates Earth's surface oxygenation. Two of the three HPQ pathways were later obtained by eukaryotes through endosymbiosis forming chloroplasts and mitochondria, enabling their rise to dominance in modern oxic ecosystems.
Keywords: biosynthesis; electron transport chain; great oxygenation event; nitrospirota; quinone.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- Raymond J., The effect of oxygen on biochemical networks and the evolution of complex life. Science 311, 1764–1767 (2006). - PubMed
-
- Zerkle A. L., et al. , Onset of the aerobic nitrogen cycle during the great oxidation event. Nature 542, 465–467 (2017). - PubMed
-
- Konhauser K. O., et al. , Aerobic bacterial pyrite oxidation and acid rock drainage during the great oxidation event. Nature 478, 369–373 (2011). - PubMed
-
- Fischer W. W., Hemp J., Valentine J. S., How did life survive Earth’s great oxygenation? Curr. Opin. Chem. Biol. 31, 166–178 (2016). - PubMed
-
- Catling D. C., Glein C. R., Zahnle K. J., McKay C. P., Why O2 is required by complex life on habitable planets and the concept of planetary “oxygenation time”. Astrobiology 5, 415–438 (2005). - PubMed
MeSH terms
Substances
Grants and funding
- 1702262/NSF (NSF)
- 1843285/NSF (NSF)
- NA/Gordon and Betty Moore Foundation (GBMF)
- 441217575/Deutsche Forschungsgemeinschaft (DFG)
- ANR-21-CE02-0018/Agence Nationale de la Recherche (ANR)
- ANR-15-IDEX-02/Agence Nationale de la Recherche (ANR)
- IDEX-IRS 2020/Grenoble-Alpes University
- EXC-2077-390741603/Deutsche Forschungsgemeinschaft (DFG)
- NA/Alexander von Humboldt-Stiftung (AvH)
- 016.Vidi.189.050/Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO)
- 18-EXXO18-0039/NASA | NASA Astrobiology Institute (NAI)
- 946150/EC | European Research Council (ERC)
- 80NSSC19K0480/NASA | NASA Astrobiology Institute (NAI)
LinkOut - more resources
Full Text Sources

