Isolation of a novel human prion strain from a PRNP codon 129 heterozygous vCJD patient
- PMID: 39977481
- PMCID: PMC11841882
- DOI: 10.1371/journal.ppat.1012904
Isolation of a novel human prion strain from a PRNP codon 129 heterozygous vCJD patient
Abstract
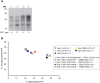
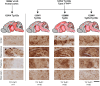
The epizootic prion disease of cattle, bovine spongiform encephalopathy (BSE), caused variant Creutzfeldt-Jakob disease (vCJD) in humans following dietary exposure. Codon 129 polymorphism of the human prion protein gene (PRNP), encoding either methionine (M) or valine (V), dictates the propagation of distinct human prion strains and up to now all but one neuropathologically confirmed vCJD patients have had a 129MM genotype. Concordant with this genetic association, transgenic modelling has established that human PrP 129V is incompatible with the vCJD prion strain and that depending on codon 129 genotype, primary human infection with BSE prions may, in addition to vCJD, result in sporadic CJD-like or novel phenotypes. In 2016 we saw the first neuropathologically confirmed case of vCJD in a patient with a codon 129MV genotype. This patient's neuropathology and molecular strain type were pathognomonic of vCJD but their clinical presentation and neuroradiological features were more typical of sporadic CJD, suggestive of possible co-propagation of another prion strain. Here we report the transmission properties of prions from the brain and lymphoreticular tissues of the 129MV vCJD patient. Primary transmissions into transgenic mice expressing human PrP with different codon 129 genotypes mainly produced neuropathological and molecular phenotypes congruent to those observed in the same lines of mice challenged with prions from 129MM vCJD patient brain, indicative that the vCJD prion strain was the dominant propagating prion strain in the patient's brain. Remarkably however, some transgenic mice challenged with 129MV vCJD patient brain propagated a novel prion strain type which at secondary passage was uniformly lethal in mice of all three PRNP codon 129 genotypes after similar short mean incubation periods. These findings establish that cattle BSE prions can trigger the co-propagation of distinct prion strains in humans.
Copyright: © 2025 Zhang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: JC is a Director and shareholder and JW is a shareholder of D-Gen Limited, an academic spin-out company (co-owners include MRC, UCL, Imperial College and the Wellcome Trust) working in the field of prion disease diagnosis, decontamination, and therapeutics. The other authors have declared no competing interests.
Figures









References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

