Supplement-Induced Acute Kidney Injury Reproduced in Kidney Organoids
- PMID: 39978331
- PMCID: PMC12342697
- DOI: 10.1159/000544795
Supplement-Induced Acute Kidney Injury Reproduced in Kidney Organoids
Abstract
Introduction: Acute kidney injury associated with the consumption of Beni-koji CholesteHelp supplements, which contain red yeast rice (Beni-Koji), has become a significant public health concern in Japan. While renal biopsy findings from several case reports have suggested tubular damage, no definitive causal relationship has been established, and the underlying mechanisms of kidney injury remain poorly understood. The complexity of identifying toxic substances in supplements containing various bioactive compounds makes conventional investigative approaches both time-consuming and challenging. This highlights an urgent need to establish a reliable platform for assessing organ-specific toxicity in such supplements. In this study, we utilized a kidney organoid model derived from adult rat kidney stem cells (KS cells) to assess the potential tubular toxicity of these supplements.
Methods: KS cell clusters were cultured in three-dimensional system supplemented with growth factors to promote kidney organoids. The organoids were subsequently exposed to Beni-koji CholesteHelp supplements or cisplatin, followed by histological and molecular analyses to evaluate structural impacts.
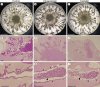
Results: Established organoids had the kidney-like structures including tubular-like structures and glomerulus-like structures at the tips of multiple tubules. Treatment with Beni-koji CholesteHelp supplements induced significant tubular damage in the organoids, characterized by epithelial cell thinning, structural disruption, and increase in cleaved-caspase 3-positive apoptotic tubular cells, similar to the organoids treated with cisplatin.
Conclusion: These findings provide the first evidence suggesting that certain toxicants in specific batches of Beni-koji CholesteHelp supplements cause direct renal tubular injury. This KS cell-based organoid system represents a cost-effective, reproducible, and technically simple platform for nephrotoxicity screening.
Keywords: Acute kidney injury; Drug-induced nephrotoxicity; Kidney organoid; Kidney stem cell.
© 2025 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Authors declare that they have no competing interests.
Figures



References
-
- Mori K, Kobayashi M, Kajita K. Evaluation of the effects of the dietary supplement containing red yeast rice on cholesterol-related index and safety: a randomized, double-blind, placebo-controlled, parallel-group study. Jpn Pharmacol Ther. 2023;51(8):1159–71.
-
- Chikasue A, Taguchi K, Iwatani R, Kimura K, Okuda S, Uesugi N, et al. Three cases of red yeast rice-containing supplement-induced acute kidney injury and Fanconi syndrome. Am J Kidney Dis. 2024:S0272-6386(24)01031-X. - PubMed
-
- Hashimoto T, Ozaki A, Hakariya H, Takahashi K, Tanimoto T. The Beni-Koji scandal and Japan’s unique health food system. Lancet. 2024;403(10441):2287–8. - PubMed
-
- Kitamura S, Sakurai H, Makino H. Single adult kidney stem/progenitor cells reconstitute three-dimensional nephron structures in vitro. Stem Cells. 2015;33(3):774–84. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

